HEALTH CARE BRIEFING: Vaccine Cold Storage in Place for Rollout
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Freezers required to store coronavirus vaccines are now in place at health systems that are preparing to administer the initial doses once the two leading candidates for shots receive a green light from regulators, federal health officials said.
The federal government will have 40 million doses—enough to vaccinate 20 million people—ready to distribute by the end of December should vaccines developed by Moderna as well as Pfizer and partner BioNTech receive emergency-use authorizations, according to Moncef Slaoui, chief adviser of the joint effort led by the Health and Human Services and Defense departments to expedite the development and distribution of coronavirus vaccines, dubbed “Operation Warp Speed.”
Pfizer and Moderna are both on the verge of submitting clearance applications to the Food and Drug Administration. The drugmakers’ shots will be distributed within 24 hours of an emergency-use authorization from the agency. Operation Warp Speed has identified hospitals, pharmacies and colleges that have cold storage capacity, and the first-available doses will initially head to these sites.
- Yesterday, Pfizer said a final analysis of clinical-trial data showed its Covid-19 vaccine was 95% effective, paving the way for the drugmaker to apply later this week for the first U.S. regulatory authorization. Pfizer and BioNTech say their vaccine protected people of all ages and racial groups, with no major safety problems so far in a trial that includes nearly 44,000 participants. Read more from Robert Langreth.
How to store and transport the vaccine is a matter of concern for states, specifically for Pfizer and BioNTech’s vaccine, which must be stored at extreme temperatures of minus-70 degrees, thus requiring special freezers. In regular refrigerators, it can only be kept for up to five days. The companies are seeking to create a second-generation version of their two-dose regimen that can withstand warmer temperatures.
Moderna’s vaccine, on the other hand, can be stored at regular refrigerated temperatures for up to a month, and be kept in ordinary freezers for long-term use, meaning it’s a more viable option for health-care facilities in the U.S. and elsewhere that lack cold-chain infrastructure.
“The states have been working with this,” said Gen. Gustave F. Perna, chief operating officer of Operation Warp Speed, said during a media briefing. Every vaccine dose will initially go to hospitals, universities, and pharmacies that have cold-chain storage capacity, to “make sure no vaccine is wasted.” Read more from Riley Griffin and Jeannie Baumann.
Biden Urges GSA to Let Transition Begin for Virus Efforts: President-elect Joe Biden said the General Services Administration’s delay in allowing the official presidential transition to begin could set back the nation’s effort to distribute a coronavirus vaccine by weeks or months. “We’ve been unable to get access to the kinds of things we need to know about—the depth of the stockpiles, we know there’s not much at all,” Biden said in a virtual meeting yesterday with front-line health workers, adding that his team doesn’t know the White House’s plan for vaccine distribution, Jordan Fabian reports.
Trump Team Promises Hospital Beds Without Moving to Slow Virus: The Trump administration has assured states they’ll have enough hospital beds and equipment to handle the alarming nationwide surge in coronavirus cases, but it isn’t advocating for additional measures to slow the virus’s spread and continues to shut out Biden’s advisers.
Several American governors have imposed new restrictions on businesses and social life, and New York City announced yesterday it would close schools. In a call with governors two days earlier, Vice President Mike Pence, who leads the federal pandemic response, said that the nation has thousands of hospital beds in reserve and ample supplies of protective gear, with vaccines around the corner. Read more from Josh Wingrove, Dina Bass and Jennifer Jacobs.
More on the Pandemic
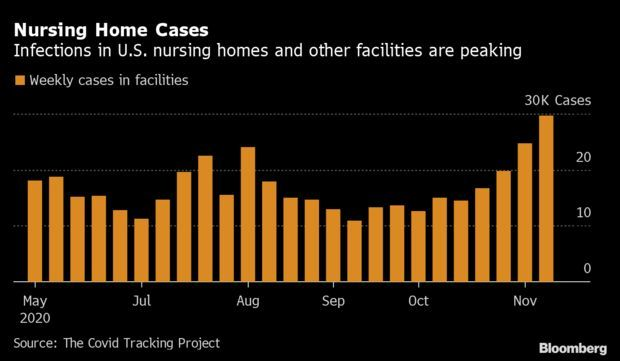
Covid-19 Ravaging Long-Term Care Centers: Covid-19 is strengthening its grip on one of the most vulnerable populations: senior citizens in long-term care. States last week reported over 29,000 new cases of Covid-19 in places like nursing homes and assisted-living facilities. Counts rose about 17% week-over-week, the sharpest hike since May, when the Covid Tracking Project began tallying the data. Half of all new nursing home cases stemmed from the viral epicenter in the Midwest, the American Health Care Association and National Center for Assisted Living reports. Ohio saw the largest last week, making up 10% of all new cases in facilities nationally.
Less than 1% of the U.S. population lives in such homes, but Covid-19 fatalities inside of them account for 40% of the nationwide death toll. Available statistics also likely fail to capture the gravity of the issue, as only 30 states report data about active outbreaks within centers. Even so, the numbers paint a stark picture of pandemic life in facilities for America’s elderly, where residents often must forgo visitation or risk further viral spread. Read more from Nic Querolo.
U.S. Approves First At-Home Test Kit: The U.S. gave a greenlight to the first Covid-19 testing kit to be used at home, adding a new tool to battle the virus as nationwide testing capabilities come under more strain. The Food and Drug Administration issued an emergency approval to Lucira Health’s rapid-result “All-In-One Test Kit,” according to a statement from the agency. It is expected to cost around $50, Lucira said, and consumers will need a prescription to acquire the kit, which provides results in under 30 minutes. Jinshan Hong and Emma Court have more.
Judge Blocks Trump Policy to Expel Migrant Children: The Trump administration must stop expelling migrant children who enter the U.S. on their own, a federal judge said yesterday, blocking an order by the U.S. Centers for Disease Control and Protection that authorized the immediate expulsion of unaccompanied minors who crossed the southern border during the pandemic. The policy led to at least 2,000 rapid deportations, according to court papers. Read more from David Yaffe-Bellany.
More Headlines:
- Lockdowns to Test Economic Endurance of Desperate U.S. Workers
- New York City Schools Close as Virus Rate Reaches 3% Trigger Point
- England Needs Tough Covid-19 Rules to Save Christmas, Adviser Warns
- Cloth Face Masks Aren’t Personal Protective Equipment, OSHA Says
Happening on the Hill
Democrats Add Billions in PPE to Relief Talks: Senate Democrats yesterday demanded that any pandemic relief bill passed in response to the worsening Covid-19 pandemic includes $11 billion for buying and creating more personal protective equipment for health-care workers. Senate Minority Leader Chuck Schumer (D-N.Y.) laid out the demands on the floor, asking for legislation to invoke the Defense Production Act to bolster domestic production of masks, gloves and other equipment for health-care providers. He said that any relief package must include $10 billion to restock the national stockpile of medical supplies, plus another $1 billion to help small businesses retool domestic facilities to produce the gear.
Senate Majority Whip John Thune (R-S.D.) said funding for protective equipment is supported by Republicans but his party supports “targeted” relief and not a “liberal wish list,” referring to the House-passed “HEROES Act” (H.R. 6800), Alex Ruoff reports. Meanwhile, White House Chief of Staff Mark Meadows, once a lead negotiator on a coronavirus stimulus package, said yesterday it’s up to Congress to proceed with those talks, even though the issue has been a “priority” for President Donald Trump.
“Obviously those discussions—if they happen—will be dictated by the House and the Senate,” Meadows told reporters when asked about stimulus negotiations after a meeting with Senate Majority Leader Mitch McConnell (R-Ky.). “We haven’t seen a real willingness by our House colleagues to look at that.” Erik Wasson and Laura Litvan have more.
Lawmakers Applaud Cancellation of Optimistic Covid-19 Ads: House Democrats praised HHS for canceling a $15 million Covid-19 ad contract they said focused on a message to “defeat despair” and “inspire hope,” rather than science. “We are pleased that following our investigation, HHS finally pulled the plug on this corrupt scheme,” according to a statement yesterday by House Oversight and Reform Chairwoman Carolyn Maloney (D-N.Y.), Catherine Larkin reports.
Daines Enters Pfizer Vaccine Trials: Sen. Steve Daines (R-Mont.) said yesterday he is participating in Pfizer’s Covid-19 vaccine trial. “My goal is to help build confidence and trust for Montanans and the American people wondering if they should take the vaccine when it is approved,” he said in a statement. Daines joins fellow Sen. Rob Portman (R-Ohio) in participating in late-stage Covid-19 vaccine trials. Portman announced earlier this week that he will be a part of human trials for Pfizer’s competitor, Johnson & Johnson.
Pot Programs Grow as House Tees Up Vote: Voters in five states gave the green light to cannabis programs on Election Day, as House Democrats could vote in the lame-duck to legalize and tax cannabis on the federal level. Nearly three-quarters of Americans now live in states that permit recreational or medical marijuana, including voters in newly added GOP-led states like South Dakota. But pot remains a federally outlawed substance, and the Senate hasn’t advanced any legislation to ease the ban. Read BGOV’s OnPoint by Michael Smallberg, Naoreen Chowdhury, and Adam M. Taylor.
Health-Related Hearings:
- The House Financial Services Subcommittee on Housing, Community Development, and Insurance plans a hearing today on the impacts of Covid-19 on insurance providers and insurance policyholders.
- The Senate Homeland Security and Governmental Affairs Committee will hold a hearing today on early, at-home Covid-19 treatment.
What Else to Know
Trump Set to Announce New Drug-Pricing Rule: Trump is set to announce a plan as soon as this week to tie U.S. government payments for drugs to lower prices paid overseas, according to a Politico report. Senior officials worked this weekend to develop an “interim final rule” that could bypass a typically months-long process of releasing a draft and seeking public feedback, Politico reports. Trump resurrected the plan after railing against Pfizer last week for releasing its vaccine data after the election, Politico said. Read more from Politico.
More Headlines:
- Opioid Use Treatment for Freed Inmates Banks on Medicaid Changes
- Trump Agency’s Ban on Medicaid Payroll Voluntary Deductions Axed
- N.D. Asks Supreme Court to Revive Pharmacy Benefit Manager Law
- Illinois Hospital Prevails in Doctor’s Legal Fight Over Lost Privileges
- Channel to Resolve Drug Discount Disputes Under Executive Review
- Galderma Gets Patent Board Reviews of Patents for Truinject Device
- PTC Therapeutics Gains FDA Fast Track Designation for Cancer Drug
- U.S. Army Settles Class Action From Discharged Veterans With PTSD
- Patient-Centered Outcomes Research Institute and HHS Plan New Efforts (GAO)
- Agencies Drug Prevention Education Grants Haven’t Fully Shown National Goal Connection (GAO)
To contact the reporters on this story: Brandon Lee in Washington at blee@bgov.com; Alex Ruoff in Washington at aruoff@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.