HEALTH CARE BRIEFING: Trump Vaccine Timeline Contradicts Aides’
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President Donald Trump said a vaccine against Covid-19 could be distributed widely to the American public as early as next month, contradicting timelines offered by many of his top health officials that ranged from the end of March 2021 to the end of that year.
“What we’ve done with the streamlining” has “been incredible,” Trump said at a news conference from the White House.
The administration’s goal is to have 100 million vaccine doses available by Dec. 31, Vice President Mike Pence said in an interview on Fox News last night
The president said earlier comments by Robert Redfield, the director of the U.S. Centers for Disease Control and Prevention, that the disbursement of the doses some time in late spring or summer of 2021 were “a mistake.” He said he called Redfield after his testimony yesterday at a Senate Appropriations subcommittee hearing to tell him that his timeline was incorrect. “I think he misunderstood the questions,” Trump said of Redfield.
But Redfield wasn’t the only top health official offering a less ambitious forecast for getting a vaccine out to the general public. Paul Mango, deputy chief of staff for policy at the Department of Health and Human Services, said it would likely be widely available by the end of next March. And Anthony Fauci, director of the National Institute for Allergy and Infectious Diseases, echoed Redfield’s timeline. Read more from Josh Wingrove, Riley Griffin and Michelle Fay Cortez.
Fauci also called Mango’s Quarter 1 timeline for full vaccine use “aspirational” in an interview yesterday. It’s more likely, Fauci said, that it would arrive toward the middle to the end of 2021. “It depends on what the vaccine is,” Fauci said.
And Trump’s timeline depends on a great many assumptions, including that the first vaccine approved for emergency use works at a high degree, that there are no major safety issues or delays within the first groups that receive it, and that the manufacturing and disbursement of the doses go off without a hitch. Read more from Josh Wingrove, Riley Griffin and Michelle Fay Cortez.
- Also yesterday, Redfield said the federal government needs an additional $5.5 billion to $6 billion to distribute a vaccine once one is approved. The CDC director said the agency doesn’t wield the resources to “support 64 jurisdictions to get this plan operational,” adding: “It’s an urgency that we get that. This is going to be a “resource-intensive” distribution, especially because some vaccines require storage at negative 80 degrees, Redfield told the Senate panel.
- Congress could give the CDC the requested funding in another coronavirus stimulus package or in a funding resolution that would need to be passed before Sept. 30. Redfield said administration officials have directed the CDC to transfer $300 million to the Health and Human Services Department for a public affairs office information campaign on the virus. That office had been run by Michael Caputo, a high-ranking HHS official and former Trump campaign aide, who took a leave of absence yesterday after reports that he posted rants on social media accusing CDC scientists of “sedition” and undermining the president. Redfield told senators that the agency wasn’t involved in the HHS media campaign beyond having the funds transferred from his agency. Related: Trump Health Spokesman Takes Leave After Social Media Rants
- The Health and Human Services Department may also need additional cash for Operation Warp Speed. “HHS has told us that to have 300 million copies of vaccine available, they need another $20 billion that they don’t have,” Roy Blunt (R-Mo.), chair of the Senate Appropriations Labor-HHS panel, said at the hearing. Read more from Shira Stein.
Shots May Be Shipped 24 Hours After Approval: Meanwhile, preparations are underway to ensure that vaccines are shipped to administration sites within 24 hours of clearance by regulators, health officials said. U.S. officials issued states new guidance designed to speed the path of Covid-19 shots to the population, said Redfield in a press conference. Read more from Riley Griffin and Josh Wingrove.
HHS Steered $700 Million From CDC
Trump administration officials pulled $700 million from the CDC to fund the Operation Warp Speed effort to develop drugs and vaccines, according to people familiar with the matter.
The money came from funds Congress appropriated to the CDC in stimulus legislation earlier this year, said the people, who asked not to be named because the matter isn’t public. The CDC received about $7.5 billion in stimulus funds this year to respond to Covid-19, on top of its annual appropriation.
CDC’s funds are being used for activities such as preparation for vaccine distribution, according to an administration official. For example, the agency last month provided funds to McKesson Corp. to distribute future Covid-19 vaccines and related supplies, the official said in an email. The CARES Act bill sent CDC funds to “prevent, prepare for, and respond to coronavirus,” the official said. Read more from John Tozzi.
Oversight Panel Says Task Force Weakened Advice: Rep. James Clyburn, (D-S.C.) chairman of the House Select Subcommittee on the Coronavirus Crisis, said in a letter to Pence he’s concerned that the White House Task Force “has weakened or retracted previous science-based recommendations” including for states that failed to follow prior guidance and are now facing serious outbreaks. Clyburn demanded that all Task Force reports tracking the spread of the virus and recommendations to contain it be made public Read more from Maria Monteros.
Also in Covid Research:
- Biden Embraces Vaccines, Science: ‘I Don’t Trust Donald Trump’
- China Expects a Vaccine to Be Ready in November, Expert Says
- NIH Launches $12 Million Minority Outreach Campaign on Virus
- Publishing Incentives Keep Scientists From Covid Collaboration
- Sorrento Gets FDA Nod for Phase 1 Covid Antibody Clinical Trial
More on the Pandemic:
- CDC Scientists Don’t Have Motives Against Trump, Redfield Says
- CDC to Publish New Guidance on Testing Asymptomatic People
- Coughing Dummies Help Boeing, Airlines Track Virus on Planes
- Nursing Homes That Cut Virus Infections to Get $2 Billion Boost
- Nursing Home Commission Recommends Safe In-Person Visitations
- Biden Says If It’s Legal He Would Sign Order to Mandate Masks
- China Made an Epic Dash for PPE That Left World Short on Masks
Also Happening on the Hill
White House Open to Boost Stimulus Offer: The White House signaled that it is willing to bolster its offer in talks with Democrats and that Republicans in the Senate should go along in order to seal a stimulus deal in the next week to 10 days. Chief of Staff Mark Meadows said the president is open to the compromise $1.52 trillion stimulus proposal from a bipartisan group of House lawmakers that was an effort to break a months-long deadlock over bolstering the U.S. economy during the pandemic. Erik Wasson, Jordan Fabian, and Laura Litvan have more.
Legislation: The House yesterday passed by a 387-33 vote H.R. 7909, directing HHS to assist states and tribes with providing child care services safely during the Covid-19 pandemic, and by voice vote S. 2683, which would create an interagency task force to assist in implementing background check requirements for child care providers.
What Else to Know
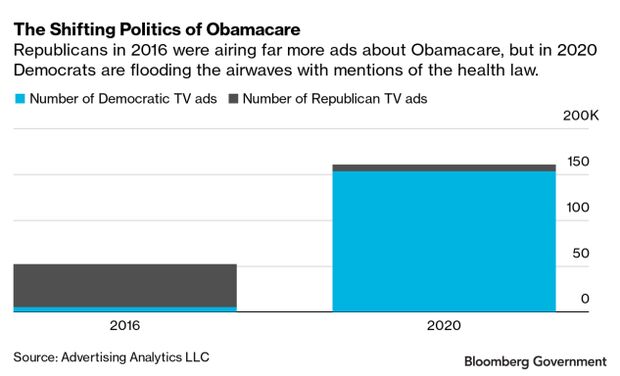
Rising Uninsured Endangers GOP Health Message: The number of Americans without health insurance has risen steadily under the Trump administration and it’s creating headaches for Republicans who once championed campaigns to roll back the Affordable Care Act. Republican leaders this week unveiled a platform that centered around defeating the pandemic and bolstering the economy, with no mention of their long-time pledge to roll back Obamacare, while Democrats are sticking with the message that won them a House majority two years ago: a promise to extend health-insurance coverage to more Americans with the ACA. Read more from Alex Ruoff.
Tillis Targeted by Drug Pricing Group: Sen. Thom Tillis (R-N.C.) will be the first target of a drug pricing group that today will launch a more than $1 million ad campaign against the Republican incumbent. Patients for Affordable Drugs Action, a drug pricing-advocacy group backed by billionaire former hedge fund managers, is targeting Tillis because he’s “the worst member of the Senate on drug pricing,” Juliana Keeping, a spokeswoman for the group, said.
Tillis has taken more than $300,000 in donations from various pharmaceutical companies this election cycle, making him one of the largest recipients of the drugmaking industry’s contributions, Federal Election Commission data show.
Patients for Affordable Drugs Action in 2018 funded campaigns against who it identified as pharmaceutical-friendly incumbents, such as Rep. Anna Eshoo (D-Calif.), who ranks among the largest recipients of industry donations in the House. Eshoo won re-election in 2018 and is up again for re-election this year, Alex Ruoff reports.
More Headlines:
- Democrats Alarmed by Georgia Waiver Request on ACA Website
- Obamacare Enrollees Can Keep Getting Premium Subsidies: IRS
- Advocates Urge EPA to Ban Sometimes Deadly Uses of Solvent
- High U.S. Drug Prices Fuel Outrage, Innovation Debate: QuickTake
With assistance from Alex Ruoff
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.