HEALTH CARE BRIEFING: Trump Plans to End Coronavirus Task Force
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President Donald Trump said yesterday that he’s looking at “phase two” of the U.S. government’s response to the coronavirus pandemic as the White House considers disbanding the task force that has helmed the efforts so far.
The group, which includes top public health experts Anthony Fauci and Deborah Birx, could be dissolved later this month, Vice President Mike Pence, who leads the task force, told reporters earlier yesterday. “Mike Pence and the task force have done a great job, but we’re now looking at a little bit of a different form,” Trump said during an event in Phoenix.
When asked whether he considered the mission of combating coronavirus to be accomplished, Trump answered: “No.”
But on Tuesday, Pence portrayed the task force as having accomplished its goal as the U.S. outbreak—the largest in the world—plateaus. There have been nearly 1.2 million cases of Covid-19 and more than 70,000 deaths from the disease, but states are beginning to try to reopen businesses and lift social-distancing regulations as the growth of the outbreak slows.
The White House has started to discuss a transition plan with the Federal Emergency Management Agency, Pence said. A possible time frame for the move could be late May or early June, he added. “We’re having conversations about that,” Pence said in a briefing the task force held for reporters yesterday, confirming an earlier report by The New York Times.
On Sunday, Trump revised upward the number of the deaths he expects from the outbreak, saying the total could reach 100,000. In April he had said he thought between 50,000 and 60,000 Americans would die. “We have slowed the spread, we have flattened the curve,” Pence said. Not every U.S. community is “out of the woods yet,” but now have the resources they need, he said. Read more from Mario Parker and Jennifer Jacobs.
Trump Pivots to ‘Phase Two’: Trump fixed his course on reopening the nation for business, acknowledging that the move would cause more illness and death from the pandemic but insisting it’s a cost he’s willing to pay to get the economy back on track. Trump shifted his rhetoric on Tuesday, removing cautionary caveats about when and whether states should reopen and instead presenting the imminent easing of stay-at-home rules as a fait accompli.
As governors across the South and Midwest have begun returning people to work, Trump said he’s pivoting to “phase two” of the nation’s response to the pandemic. “Will some people be affected? Yes. Will some people be affected badly? Yes,” Trump said. “But we have to get our country open and we have to get it open soon.” Read more from Jordan Fabian and Mario Parker.
Related: Most States Fall Short of White House Reopening Criteria
Register for the BGOV Webinar Today at 2 p.m.: Join Bloomberg Government as we examine health care policy during a global pandemic. This live discussion will cover the continuing response to the coronavirus and the implications for long-term health policy. Register here.
Congressional Virus Efforts
HHS Appropriations Hearing: House appropriators will hold a bipartisan, if lonely, hearing on the government’s response to the coronavirus today, Labor-HHS-Education Subcommittee Chairwoman Rosa DeLauro (D-Conn.) said. “It will be bipartisan,” DeLauro told The Hill on Monday, adding that the ranking member of the subcommittee, Tom Cole (R-Okla.), and others will be in attendance, Jack Fitzpatrick reports.
DeLauro’s assurance contrasts with the White House’s decision to keep Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, from testifying in front of the Democratic-controlled House panel, while allowing him to testify to the Republican-controlled Senate. Trump told reporters he made the decision “because the House is a set-up. The House is a bunch of Trump haters.” Fauci won’t be the only one absent. Some panel members won’t attend in person and will get other members to ask questions for them, said DeLauro.
However, Fauci is set to testify at a Senate Health, Education, Labor, and Pensions Committee hearing on May 12, according to Evan Dixon, press secretary for Chairman Lamar Alexander (R-Tenn.). He will be joined by CDC Director Robert Redfield, FDA Administrator Stephen Hahn, and Health and Human Services Assistant Secretary Brett Giroir.
DeLauro’s focus at the hearing will be more on the present emergency than looking far into the future, she said. “At the moment, we need answers right now of where we are in this process, and that’s what this hearing will be focused on,” she said. “How do we best tackle where we are now? How do we save lives, having lost 68,000 or more lives? How do we prevent that further loss of life?”
Cole has advocated for boosts to funds for the National Institutes of Health and the CDC, but said in April the coronavirus highlights the need to do more. NIH funding increased 30% from fiscal 2014 to fiscal 2019, and appropriators launched the Infectious Disease Rapid Response Reserve Fund in fiscal 2019, for example. “All of that and it wasn’t enough,” Cole said. “We got overwhelmed in 12 weeks.”
DeLauro and House Appropriations Chairwoman Nita Lowey (D-N.Y.) said in a joint statement May 1 that in the short-term, Congress needs to know what officials are doing “on surveillance, testing, contact tracing, quarantining, social distancing, and the production and distribution of personal protective equipment.” Medium and long-term goals include understanding “the viability of therapeutics and vaccines in development” and making sure “lasting investments in our public health infrastructure are made instead of reacting to public health crises when they arise,” she said.
- Meanwhile, Speaker Nancy Pelosi (D-Calif.) is pushing Democrats to get out of the gate quickly with another multibillion-dollar virus stimulus package to give the House more leverage in talks with Senate Republicans, who are seeking to apply the brakes on any new round of expansive relief. Pelosi’s strategy of ensuring that the next economic measure originates in the House, unlike the prior $2 trillion version, was underscored in a memo to Democrats yesterday from Lowey. “In the coming days, House Democrats will release our full proposal for the next phase of relief,” Lowey wrote.
- Trump is pushing his own set of counter demands, including changes to tax law, that would complicate negotiations on an eventual stimulus. “Well run States should not be bailing out poorly run States, using CoronaVirus as the excuse!” Trump tweeted yesterday. “The elimination of Sanctuary Cities, Payroll Taxes, and perhaps Capital Gains Taxes, must be put on the table,” he said. “Also lawsuit indemnification & business deductions for restaurants.” Billy House and Erik Wasson have more.
- Senate Minority Leader Chuck Schumer (D-N.Y.) urged the Trump administration to develop a comprehensive, nationwide plan by May 24 to make sure states have sufficient testing to begin to safely reopen. It is the Trump administration’s responsibility to establish a strategy as fast as possible and address other problems, including managing the supply chain and analyzing national data, Schumer and 40 other Senate Democrats wrote in a letter to Trump yesterday, Kim Chipman reports.
Hospitals Say Aid Not Enough for Lost Revenue: The country’s hospitals say they are losing more than $50 billion per month largely from canceled surgeries and want Congress to inject more public funds into the industry. The American Hospital Association unveiled a report yesterday projecting health-care facilities will lose $202.6 billion between March and June, more than the $175 billion that Congress has approved in relief for doctors and hospitals this year. They asked lawmakers to do more for the industry, including finding a new way to cover the uninsured. Read more from Alex Ruoff.
Democrats Challenge Pandemic Watchdog: Senate Democrats challenged a vow of “fairness and impartiality” by Brian Miller, Trump’s nominee to oversee trillions of dollars being spent in the effort to rescue the economy from the coronavirus pandemic. Democrats have questioned Miller’s ability to serve as Special Inspector General for Pandemic Recovery in light of his current post, as a White House lawyer who participated in Trump’s impeachment defense. Miller at the hearing said he would let Congress know if he was pressured in his new role. Read more from Saleha Mohsin and Laura Davison.
Pandemic Dominates Hearing on Spy Chief Pick: Rep. John Ratcliffe (R-Texas) told senators one of his top priorities if he’s confirmed as the nation’s spy chief will be gathering intelligence on the origin of the coronavirus outbreak. Ratcliffe gave the pledge during a confirmation hearing on his nomination by Trump to serve as director of national intelligence. It was the first hearing on any topic for a Senate returning to work while adjusting to the need for social distancing in the pandemic. Senate Intelligence Committee members appeared in shifts to ask questions, many with masks under their chins when they spoke.
Read more: Trump Pushes Virus-From-China-Lab Theory That Divides U.S. Spies
Amid increasing pressure by the administration for U.S. intelligence agencies to blame China for the scale of the pandemic that began there, Ratcliffe, a former federal prosecutor, said that he would ensure “the intelligence community will be laser-focused on getting all of the answers that we can regarding how this happened, when this happened.” Chris Strohm and Steven T. Dennis have more.
Letters & Legislation:
- Murray Floats Shifting Unemployed Workers to Health Workforce
- Azar Urged to Secure Better Testing at Prison Quarantine Sites
- Lawmakers Seek Pandemic-Related Behavioral Health Funding
- Congressman Sues to Define Limits of Michigan Governor’s Power
- Nursing Homes Industry Group Asks HHS, FEMA for $10 Billion
Testing, Prognoses & Reopening
America Tiptoes Toward Reopening: Trump’s push to reopen state economies would have Americans self-policing their behavior even as projections indicated that shopping, eating out and getting post-quarantine haircuts may lead to even more deaths. Two recent estimates, one by Trump, have predicted that the toll from the crisis will reach or surpass 100,000 deaths. Despite the grim forecasts, states continued to move toward resuming commerce, impelled by Trump, business people and citizens weary of lockdowns without an end game.
The White House has released loose guidelines for a return to economic life that put the onus of decision-making on state governors. Florida, Georgia and Texas, whose leaders have bridled at restraints, saw their residents stirring at stores and restaurants. Meanwhile, the governors of Ohio, Arizona, Rhode Island and California have announced more cautious plans to slowly reopen businesses in a limited fashion to avoid human contact.
- The states dealing with the worst economic fallout from the coronavirus, by one measure, are taking a careful approach to their reopenings to protect public health. Among those with the 10 highest claims for unemployment benefits as a portion of their population, Georgia was the first to reopen its economy and Gov. Brian Kemp (R) let a “shelter-at-home” order for most residents expire as of Friday, Vivek Shankar reports.
It’s not yet clear how the U.S. public is responding to more economic freedom absent a national safety net. A Washington Post-University of Maryland survey released this morning shows that most Americans clearly oppose the reopening of restaurants, retail stores and other businesses. The majorities of those polled expressed fears that they could become infected by the coronavirus, as well as a belief that the worst of the crisis isn’t over. Read more from Margaret Newkirk and Emma Court.
More Headlines:
- U.S. Virus Cases Rise 1.9%, Slower Than Past Week’s 2.6% Average
- Texas Hospitalizations Rise 23%, Most in More Than Three Weeks
- White House Says Johns Hopkins Study Based on Faulty Assumptions
- Azar Says He Hasn’t Seen Report Showing 200k Deaths by June 1
- Former FDA Chief Gottlieb Says Virus Cases Aren’t Falling as Hoped
Research, Treatment & Coordination
Gilead Treatment Rekindles Push to Rein In Prices: Gilead Sciences, maker of the novel coronavirus treatment remdesivir, faces a challenge from advocates of drug-pricing controls who want to set an example for the pharmaceutical industry. Some lawmakers in Congress and advocacy groups aligned with Democrats are making the case to include in the next Covid-19 response package provisions to deny drugmakers such as Gilead exclusive rights to treatments and vaccines for the virus, as well as require price transparency for companies bringing new medicines to market.
Gilead, with a history of contentious pricing, is a prime example of the need to rein in prices now, advocates of controls say. “We’re lifting up Gilead,” Margarida Jorge, national campaign director of Lower Drug Prices Now, told reporters yesterday. “It’s certainly not the only example but it is certainly at this moment a high-profile one.” Read more from Alex Ruoff.
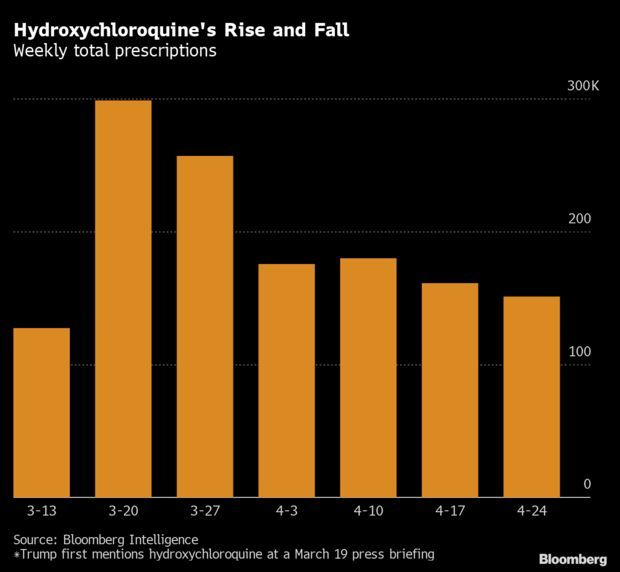
Trump’s Malaria Drug Tout Cost Millions: Trump has stopped talking about the decades-old antimalarial drug that he once touted as a “game changer” for Covid-19, but it won’t be as simple for the rest of the health system to just move on. When Trump first began touting the drug in mid-March, a frenzy ensued as hospitals, patients and doctors raced to secure supplies. Many believed even if the drug didn’t turn out to be an effective coronavirus treatment, it might be able to ward off infection.
But as quickly as pharmacies were drained of the pills, the tide has now turned against hydroxychloroquine and its chemical cousin, chloroquine. Regulators and scientists have raised concerns about potentially serious side effects. But the surge resulted in shortages that left patients who had long taken the drug for treating lupus and arthritis hunting for alternatives. Hydroxychloroquine prescriptions jumped to 298,660 during the week of March 20, according to a Bloomberg Intelligence analysis. But prescriptions have now plummeted back to nearly normal levels.
The swift embrace and rapid abandonment of hydroxychloroquine underlines how publicity of evolving science can have unpredictable consequences on the behavior of physicians and patients. The episode also shows how supply chains and government agencies struggle to keep up with such changes, especially when one perspective is amplified by the president. Anna Edney has more.
Pfizer Starts U.S. Trials of Experimental Vaccine: Pfizer has administered the first U.S. patients with its experimental vaccines to fight the disease caused by the novel coronavirus, part of a bid to shave years off of the typical time it takes to develop a new inoculation. The trials are being conducted at the New York University Grossman School of Medicine and the University of Maryland School of Medicine, the drugmaker said yesterday. Read more from Cynthia Koons.
Covered Patients May Face Steep Virus Bills: Covid-19 patients with short-term health plans—which typically have restrictions on coverage—could find themselves on the hook for tens of thousands of dollars. The HHS has said the government will pay for coronavirus testing—but not treatment—of people with short-term plans. Trump has touted the health plans as a more affordable alternative to Obamacare, even though they may lack the coverage protections of policies created under the Affordable Care Act.
Many short-term plans have limits on what they’ll pay for hospital coverage, as well as overall yearly maximums.The plans usually cost less than comprehensive plans, but people may not be aware of their limitations until they incur medical costs that aren’t covered. Read more from Sara Hansard.
- Humana to Waive Patients’ Share of Some Costs for Rest of 2020
- Barr urges Trump administration to back off call to fully strike down Obamacare (CNN)
HHS Waits for FDA as It Buys ‘Reusable’ N95s: The Department of Health and Human Services bought 10 million “reusable” N95 respirators on condition that those masks receive emergency use authorization by the FDA, according to an HHS spokesperson. The contract with the medical supply distributor American Medical Depot lists the respirators as having a 14-day reusability. However, the respirators—made by Nexera Medical—are only approved for single use by the Food and Drug Administration, American Medical President Akhil Agrawal said in an emailed statement. Read more from Shira Stein.
More Headlines:
- Protect Patient Privacy When Inviting Media Coverage, HHS Says
- Covid-19 Research Under Hacker Attacks, U.S. and U.K. Warn
- Regeneron Covid-19 Antibody Treatment Could Be Out by Fall
- Symptoms May Have Started Appearing in Europe in Early December
- Employers Can Bar Workers With Medical Issues During Pandemic
What Else to Know
HHS ‘Disappointed’ Ousted Official Has Not Shown Up to Work: A spokesperson for the HHS says former Biomedical Advanced Research and Development Authority Director Rick Bright has not appeared at the NIH, where he was reassigned to work on diagnostics testing for COVID-19. Bright, who wants to remain BARDA director, was moved to NIH after he disagreed with his supervisors at the Department of Health and Human Services over the seriousness of the threat of the novel coronavirus, shortages of protective gear and contracts that went to companies with political connections, reports Anna Edney.
- Rep. Anna Eshoo (D-Calif), chairwoman of the House Energy and Commerce Health Subcommittee, yesterday announced plans to hold a hearing on Bright’s whistleblower complaint next week.
Doctors Hope Relaxed Telehealth Rules Continue: Doctors relying on eased telehealth rules to treat patients during the coronavirus pandemic could face familiar obstacles to remote treatment when enforcement of security and privacy requirements return. Chatting with physicians online has drastically expanded during the pandemic via video-conferencing apps such as Google Meet, Zoom, Skype, and Apple FaceTime. That was more difficult when privacy and security rules were more rigorously enforced. Ayanna Alexander has more.
Justices Hear HIV/AIDS-Tied Case: Another pandemic was the focus of U.S. Supreme Court arguments yesterday morning: the HIV/AIDS pandemic. On day two of the high court’s unprecedented livestream oral arguments, the justices seemed poised to strike down a requirement that foreign entities adopt an explicit policy against prostitution and sex trafficking in order to receive federal funding to fight the pandemic abroad, Kimberly Strawbridge Robinson report.
Trump Administration Sued by Migrants’ Kids for Denied Virus Aid: The Trump administration was sued over a provision in the coronavirus relief package that bars U.S.-citizen children of undocumented immigrants from getting stimulus payments. A group of seven children and their parents claim the law violates the children’s constitutional rights. The Trump administration also is being sued by citizens denied virus aid because they are married to undocumented immigrants. Read more from Bob Van Voris.
More Headlines:
- Stem Cell Funding Backers Amass Ballot Signatures Despite Virus
- Aurinia Gets an Outperform at Cowen on Lupus Drug Potential
- AbbVie Wins U.S. Antitrust Nod for Botox Maker Allergan
- Medicare Low-Income Pay Adjustment Calculation Rule Vacated
- Henry Ford Heath Defeats Medical Technician’s Race Bias Suit
To contact the reporters on this story: Brandon Lee in Washington at blee@bgov.com; Jack Fitzpatrick in Washington at jfitzpatrick@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Zachary Sherwood at zsherwood@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.