HEALTH CARE BRIEFING: The Drugs Targeted in Democrats’ Big Bill
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Democrats are advancing a plan to negotiate the prices of drugs Medicare spends the most on, potentially saving billions of dollars annually. Medicine from Merck, Johnson & Johnson, AstraZeneca, and other major drugmakers could be targeted.
Democrats reached a compromise on their drug proposal that would allow the government to negotiate prices of medicines at least nine years out from their initial approval date. That means new drugs, which can make headlines with high sticker prices, wouldn’t be affected.
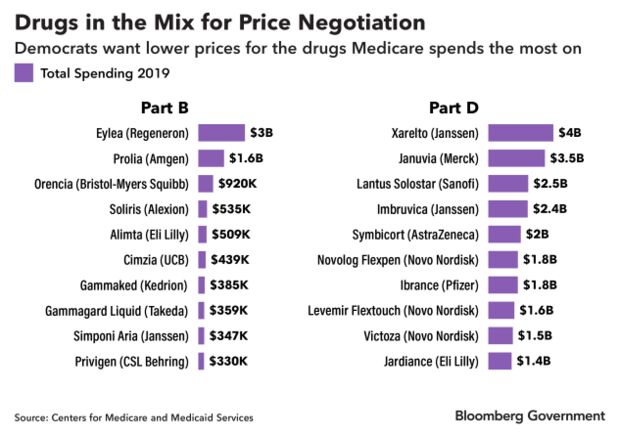
Nearly half of the prescription pharmaceuticals that were the top 10 costliest to Medicare’s outpatient drug and pharmacy benefits in 2019 could face negotiation, according to a Bloomberg Government analysis. Medicine administered in a doctor’s office is paid for under Part B of Medicare, and drugs obtained through a pharmacy are paid for under Part D.
The plan is part of Democrats’ sweeping social spending and tax package (H.R. 5376) House leaders plan to vote on before Thanksgiving. It could still face changes before it becomes law. “We’re coming out of the gate negotiating over the most expensive drugs: we’re talking cancer, arthritis, anticoagulants,” Senate Finance Chair Ron Wyden (D-Ore.) told reporters last week. “That’s a significant change.”
The White House estimates the proposal would reduce government spending by about $100 billion over 10 years.
Bloomberg Government identified 20 drugs in the latest Medicare data that would most likely be affected, though no formal selection has been made. The proposal wouldn’t take effect until 2025, so the makeup of drugs eligible for negotiation could change.
The secretary of Health and Human Services would pick 10 drugs out of the 100 costliest drugs for price talks with the manufacturers under the current proposal. Drugs approved in the last nine years, or 13 years for complex biologic drugs, would be excluded. The package would exempt orphan drugs, which treat rare diseases with narrow indications.
Many of the drugs that cost Medicare $1 billion or more in 2019 are at least a decade old and have a single manufacturer, meaning they have no cheap generic alternative. The proposal is aimed at bringing the prices of those drugs down. Read more from Alex Ruoff, Jasmine Ye Han, and Valerie Bauman.
Related:
- BGOV OnPoint: House Reconciliation Plan Awaits Vote
- BGOV OnPoint: Deadlines, Biden Economic Plan Top Year-End Agenda
The Coronavirus Pandemic
Pfizer Asks U.S. to Make Boosters Available to All Adults: Pfizer said it had asked U.S. regulators to expand access to its Covid-19 booster shot to every adult and submitted data from a large trial showing that an additional dose is safe and highly effective at preventing infection. The drug giant and partner BioNTech said in a statement yesterday they had requested that the Food and Drug Administration amend the shot’s existing emergency-use authorization and extend eligibility for third doses to everyone 18 and up. Findings from the trial demonstrated a relative vaccine efficacy of 95% when compared with those who didn’t get a booster, the companies said.
The FDA will review the authorization request as expeditiously as possible and determine whether to hold a meeting of its vaccines advisory panel following an initial evaluation of the information submitted, an FDA spokesperson said in an email. Regulators first cleared a Pfizer booster shot in September for people 65 and older; individuals 18 to 64 at high risk of developing severe Covid-19; or adults whose work or living conditions put them at high risk of serious complications from the disease. The FDA and its advisers signed off on this decision after evaluating clinical trial data that showed the booster prompted a strong immune response. Riley Griffin has more.
- People who are fully vaccinated are 16 times less likely to end up in an ICU or to die from Covid-19 than those who aren’t immunized, an Australian government study found. Nearly 16 out of 100,000 people who had yet to receive a Covid-19 vaccine landed in intensive care or died after contracting the virus, compared to fewer than one in every 100,000 who were fully vaccinated, data compiled by health authorities in New South Wales, Australia’s most populous state, found. Read more from John Liu and Dong Lyu.
- Meanwhile, Merck said the U.S. committed to buy 1.4 million courses of its Covid-19 pill made with Ridgeback Biotherapeutics for about $1 billion, bringing the country’s total to 3.1 million. The purchases are contingent on Food and Drug Administration clearance of the oral antiviral, called molnupiravir, Merck said in a statement, and the U.S. has the ability to buy 2 million more courses. Pills from Merck and Pfizer have both shown effectiveness in keeping high-risk patients out of hospitals. Read more from John Lauerman.
Vaccinations Can Help Avert Holiday Spike, Fauci Says: Stepping up the rate of Covid-19 vaccinations and booster shots can help skirt a holiday surge in new cases that have dropped to a plateau of about 70,000 a day, White House medical adviser Anthony Fauci said. While deaths and hospitalizations have fallen in the U.S., it’s far too early to pull back on mitigation measures such as mask-wearing, Fauci said in an interview on Bloomberg Television. Fauci pointed to a recent resurgence in Europe as a warning that the virus could begin rising again in the U.S., and that increased use of vaccines are key to keeping cases and deaths at bay. Read more from Fiona Rutherford.
Businesses Argue for Emergency Relief From Shot-or-Test Rule: Several affiliated businesses told a federal appeals court that their operations will suffer harm that can’t be fixed unless they get emergency relief from the Biden administration’s rule requiring large employers to mandate Covid-19 vaccination or regular testing of workers. The businesses, led by management company BST Holdings, asked the U.S. Court of Appeals for the Fifth Circuit yesterday to issue an injunction blocking the regulation. Failure to do so, they told the court, would make them unable to hire workers and place them at a disadvantage against smaller companies that aren’t subject to the rule. Read more from Robert Iafolla.
More Headlines:
- BioNTech Raises Covid-19 Vaccine Forecast to Nearly $20 Billion
- Judge Sides With United on Vaccine Mandate, But Is ‘Disturbed’
- COP26 Host Scotland Weighs Tighter Covid-19 Rules as Cases Rise
- EU Set to Approve Regeneron-Roche Combo for Covid-19: Reuters
What Else to Know Today
GOP Doctors Press Biden on Surprise Billing Rule: The GOP Doctors Caucus is the latest group in Congress to urge the Biden administration to reverse course on its surprise billing regulations. Members sent a letter to HHS Secretary Xavier Becerra and others yesterday seeking major revisions to how billing disputes between doctors and insurers are settled. The interim final rule implementing the No Surprises Act “does not reflect legislation that could have passed Congress or the law as written” and “immediate revisions are necessary,” the lawmakers, including Rep. Michael Burgess (R-Texas), said in their letter.
- The letter comes the same week over 150 other lawmakers also criticized how the interim final rule was crafted. The IFR may “incentivize insurance companies to set artificially low payment rates,” which would “narrow provider networks and jeopardize patient access to care,” the lawmakers said. Read their letter here.
- The American College of Emergency Physicians endorsed the letter from earlier this week, saying it and “leaders throughout the medical community” are “deeply concerned that the IFR as written is a drastic departure from the legislation passed by Congress last December to resolve billing disputes fairly and without bias through an independent dispute resolution process.” Read their statement here.
J&J Persuades Oklahoma Top Court to Toss Opioid Award: Oklahoma’s Supreme Court threw out a $465 million opioid award against Johnson & Johnson after finding a judge wrongfully concluded the drug maker violated state law with its marketing campaigns. The state’s highest court yesterday ruled Judge Thad Balkman misconstrued Oklahoma’s public-nuisance law in ruling in 2019 J&J’s marketing of its painkillers helped fuel the state’s opioid epidemic. He concluded J&J should pay hundreds of millions to fund treatment and other social-services programs. Oklahoma is one of many states that have sued opioid makers like J&J under public-nuisance law. Read more from Jef Feeley.
More Headlines:
- FCC Awards Another $42 Million for Pandemic Telehealth Program
- Washington’s First-in-Nation Long-Term Care Fund Draws Lawsuit
- N.J. PATH Settles DOJ Job Bias Lawsuit Over Medical Exams, Probes
- J&J Loses Bid for Agency Review of Alcon’s Eye Surgery Patents
Editor’s Note: Bloomberg Government’s Health Care Briefing will not publish tomorrow during the Veterans Day federal holiday. We’ll resume publication Friday, Nov. 12.
With assistance from Alex Ruoff
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.