HEALTH CARE BRIEFING: Pentagon Data Shows PFAS in Nearby Water
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Newly released Defense Department data reveals that its decades-long use of “forever chemicals” has contaminated the drinking water in some nearby communities—in some cases significantly.
The DOD recently, for the first time, posted online data showing off-base concentrations of per- and polyfluoroalkyl substances, or PFAS, that resulted from its activities on military bases, formerly used defense sites, and National Guard facilities. Data isn’t available for many states, and the department didn’t immediately reply on Tuesday to questions about its timeline to release more information.
“These numbers are extremely high and alarming,” said Scott Faber, who directs government affairs for the Environmental Working Group (EWG). The group’s analysis of the defense department’s newly released and other information provides “further evidence that the DOD has failed service members and as importantly the defense communities that support them,” he said.
The seven states where DOD has facilities and has released off-base drinking water data for more than a dozen sites are Florida, Pennsylvania, Montana, Michigan, Wisconsin, Washington, and Virginia. Congress ordered the department to release the data in the National Defense Authorization Act for Fiscal Year 2022 (Public Law 117-81).Read more from Pat Rizzuto.
Health Deals on Pace Despite Interest Rates
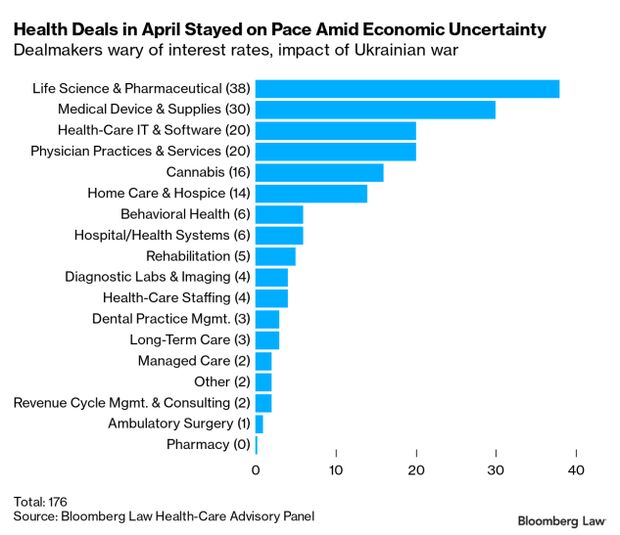
Health-care mergers and acquisitions continued at a steady pace in April in the face of rising interest rates and ongoing uncertainty from the Russian invasion of Ukraine. The total number of transactions for the month was 176, up slightly from 174 in March, but down from 207 deals in April 2021.
The top four sectors were life sciences, medical devices, physician practices, and healthcare IT, each with 20 or more deals in April. Other areas with significant deal volume included the cannabis sector and the home care and hospice sector. KPMG in Washington and FocalPoint Partners of Chicago prepared the curated year-to-date list and list of select April transactions for Bloomberg Law. Read more from Christopher Brown.
What Else to Know Today
Biden to Meet with Formula Manufacturers: President Joe Biden Wednesday “will meet virtually with infant formula manufacturers to discuss his administration’s progress to accelerate infant formula production and imports of formula through Operation Fly Formula,” according to White House daily guidance, Chelsea Mes reports.
Environmental Health Equity to Get Boost With New Office: A new office within the Health and Human Services Department will develop a department-wide strategy to protect disadvantaged communities from environmental health issues. The Office of Environmental Justice, unveiled Tuesday, will work with other agencies on the issue, including assisting the Office for Civil Rights with ensuring compliance with civil rights laws. The new office will be part of HHS’s Office for Climate Change and Health Equity. Read more from Shira Stein.
Seizure Drug Deemed Controlled Substance by DEA: A seizure treatment drug approved by the Food and Drug Administration will be categorized as a controlled substance, the Drug Enforcement Administration announced Tuesday. The general public will have the next month to weigh in on the DEA call, which would require require hospitals, distributors, manufacturers, and others on the supply chain behind the seizure drug ganaxolone to register with the DEA, keep records, and abide by other agency rules, according to the agency’s interim final rule. Read more from Ian Lopez.
Agency Vacancy ‘Loophole’ Ruling Could Echo Across Government: A statutory “loophole” for keeping temporary government leaders in place was validated last week, as a federal appeals court ruled the application of a law intended to limit the time a temporary official can serve in a Senate-confirmed position is “vanishingly small.” The ruling, which stemmed from a surgical device patent dispute involving Arthrex, confirmed the power of interim agency directors, with the US Court of Appeals for the Federal Circuit saying the scope of the 1998 Federal Vacancies Reform Act is extremely limited. Read more from Samantha Handler.
More Headlines:
- Prenatal Genetic Testing Suit Bounced by Rhode Island Top Court
- Special Rule for Medical Cases Said Inapplicable in Federal Courts
- Aetna Settles Suit Over Residential Mental Health Care Coverage
- Sanofi Hemophilia Drug Gets Breakthrough Designation From FDA
- Data Gaps Hinder Understanding of ‘Skinny’ Plans’ Pandemic Role (GAO)
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editor responsible for this story: Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.