HEALTH CARE BRIEFING: Moderna Vaccine Gets FDA Panel Endorsement
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Moderna’s Covid-19 vaccine won backing from a panel of experts who advise U.S. regulators, setting the stage for its shot to be the second vaccine cleared in the U.S.
Food and Drug Administration advisers voted 20 to 0, with one abstention, yesterday that the benefits of the vaccine outweigh any risk, delivering a key boost to efforts to ramp up the U.S. immunization campaign. The effort is initially focusing on health-care workers and old people living in long-term care facilities.
Last night, the FDA said it will rapidly work toward finalization and issuance of an emergency use authorization, which could come as early as today. The agency has also notified the U.S. Centers for Disease Control and Prevention and Operation Warp Speed, so they can execute their plans for timely vaccine distribution, Hari Govind reports.
The FDA’s advisory panel met a week ago on Pfizer and BioNTech’s vaccine and voted 17 to 4, with one abstention, that the vaccine’s benefits outweighed its risks. The FDA doesn’t have to follow its advisers’ recommendations, although it often does and did in that case, authorizing the vaccine last Friday, a day later. It’s likely the FDA will move at the same pace for Moderna.
Moderna’s product is based on the same technology as a shot made by Pfizer and BioNTech, and like that vaccine, showed a high degree of efficacy in late-stage trials that surprised even health experts. “To go from having the virus sequenced in January, to having two vaccines in December is a remarkable achievement,” said James Hildreth, a member of the panel who is also president of Meharry Medical College.
- One advantage the Moderna vaccine wields over Pfizer’s is easier storage requirements and smaller shipment batches, a potential boon especially in rural areas where hospitals may not have the specialized freezers needed to store Pfizer’s shot. Where Pfizer’s shot needs to be stored in ultra-cold freezers long term and must be used within five days of thawing, Moderna’s option may be stored in a regular refrigerator for up to one month.
- Most rural facilities in Colorado opted for the Moderna vaccine because it was easier for them to store, according to Michelle Mills, chief executive officer for the Colorado Rural Health Center. Still, rural providers who opted for Pfizer’s vaccine haven’t reported had any issues with storage so far, she said. Read more from Jacquie Lee.
In an analysis ahead of the meeting, FDA staff determined the Moderna shot is 94.1% effective against symptomatic Covid-19. The report listed side effects like headache, fatigue and muscle aches, which are typical of a vaccine.
FDA, Pfizer Revising Vaccine Side Effect Guidelines: At the panel meeting yesterday, some of its discussion focused on a handful of allergic reactions experienced by people in the U.S. and U.K. who took Pfizer’s shot. Side effects seen in the rollout of the Pfizer-BioNTech shot are now leading federal regulators to revise instructions for doctors and those that take the shot. Pfizer and the FDA are revising fact sheets distributed with the doses, according to Doran Fink, deputy chief of FDA’s division on vaccines, at the advisory panel’s meeting. Read more from Anna Edney.
Pfizer, U.S. Wrangle Over Distribution Pace: Pfizer pushed back on claims it is experiencing problems producing its vaccine, as the company and the federal government continued to try to reach agreement that would eventually double the number of doses available for the U.S.’s vast immunization effort. Operation Warp Speed’s chief scientist, Moncef Slaoui, said that the U.S. is close to a deal for another 100 million vaccine doses, but said they wouldn’t arrive until the second quarter of 2021.
HHS Secretary Alex Azar is likely to be vaccinated next week, a person familiar with the matter said. He and other top HHS officials would prefer to get the Moderna vaccine rather than the Pfizer one partly because of the dispute between Pfizer and the administration, one official said. They also want to highlight Moderna in part because it, unlike Pfizer, was also a full participant in Operation Warp Speed. Read more from Riley Griffin and Josh Wingrove.
- Azar to Stay on Job After Wife Tests Positive: Meanwhile, Azar’s wife, Jennifer, has tested positive for the coronavirus, but he will continue going to work and spoke maskless at a pair of events a day before her case was confirmed. Read more from Josh Wingrove and Jennifer Jacobs.
Fears Prompt Some Medical Workers to Balk at Getting the Vaccine: Some nurses and emergency-response workers have expressed reluctance to take the new coronavirus vaccine, a reflection of unease that U.S. officials hope to overcome as they ramp up the nationwide immunization effort.
For months, surveys showed widespread skepticism about the vaccine after the Trump administration’s push to get it out before the November election. Public-health authorities say people’s concerns have eased since then, but this week’s launch of vaccinations made it clear that some health-care workers and first responders remain unwilling to get the shot. Read more from Shruti Date Singh, Henry Goldman and Angelica LaVito.
Congress to Get Limited Vaccines: Congress will be receiving a “small number” of Pfizer vaccine doses to “to meet long-standing requirements for continuity of government operations,” Attending Physician Brian P. Monahan said in a statement yesterday. Monahan said members should call his office to schedule appointments, and that once members were complete, doses would be offered to other essential staff in an order to be determined in the coming weeks.
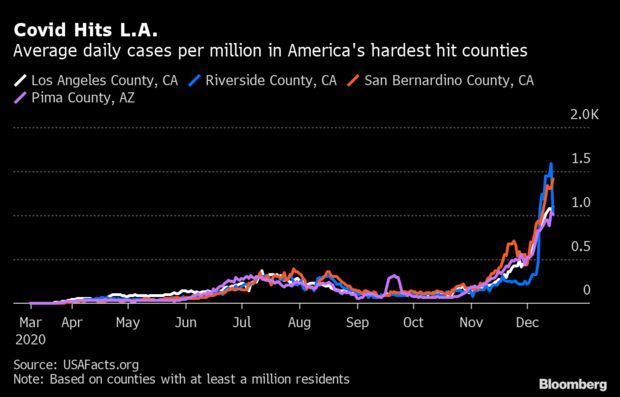
Greater Los Angeles Now Worst-Hit in U.S.: Greater Los Angeles is emerging as America’s worst-hit metropolitan area as Covid-19 rips across California at an unprecedented rate. The past week saw San Bernardino and Los Angeles counties recording 1,415 and 1,102 average daily cases per million residents, respectively, the highest rates among counties of at least a million people, according to USAFacts, a nonprofit aggregator of government statistics used by the CDC. Jonathan Levin and Christopher Palmeri have more.
More Headlines:
- CVS, Walgreens Vaccine Offers Shunned by Some Nursing Homes
- Regeneron Antibody Cocktail Treatment Shows Promise in Report
- Lawmaker, Biden Adviser Richmond Tests Positive for Covid-19
- Europe Leaders Isolating After Macron Tests Positive for Covid-19
Happening on the Hill
Lawmakers Are Facing Down Deadline on Aid Plan: The federal government faces the possibility of a partial weekend shutdown because of disputes over some of the details in a pandemic relief plan, Sen. John Thune (R-S.D.) said. Congress still doesn’t have legislation for the relief plan, which leaders plan to attach to a $1.4 trillion bill that would fund the federal government through the end of the fiscal year on Sept. 30. The government is currently operating on stopgap funding that expires today. If the House and Senate need more time to process the legislation, another short-term funding bill would be needed.
But Thune, who is the chief GOP vote-counter in the Senate, said some lawmakers, who he didn’t name, may object in order to push their own priorities in the bill. That would leave the government without funding, though the White House budget office has legal discretion to avoid the start of federal worker furloughs if funding is likely to pass in the near future. Read the latest on negotiations from Bloomberg News.
Democrats Asks About Covid-19 Misinformation: Sens. Amy Klobuchar (D-Minn.), Gary Peters (D-Mich.) and Jack Reed (D-R.I.) sent a letter this week to Biden’s transition team urging them to create a Covid-19 misinformation and disinformation task force based on legislation (S. 4499) they introduced this Congress. It would coordinate the government’s response to false Covid-19 and vaccine information.
Sackler Denies Improper Purdue Transfers: Former Purdue Pharma Director David Sackler denied claims his family improperly shifted billions of dollars from sales of the controversial opioid-based painkiller OxyContin to offshore accounts. Sackler, son of ex-Purdue President Richard Sackler, told the House Oversight and Reform Committee only a “very small amount” of money moved out of the family-owned drug company by the Sacklers ended up in foreign banks, and those transactions shouldn’t raise questions. Jef Feeley has more.
What Else to Know Today
Youth Tobacco Use Falls, CDC Finds: Kids aren’t turning to tobacco as often, even shunning the popular e-cigarettes, and that started even before the quarantining and lockdowns in the U.S., according to a new report from the CDC released yesterday. The number of middle- and high-school students who use tobacco fell 28% in 2020 to about 4.5 million, according to the report, which used survey data collected from Jan. 16 to March 16. Read more from Richard Clough and Tiffany Kay.
Foreign Influence Threats Prompt Changes to NIH: Biomedical researchers would have to disclose foreign collaborations and other non-monetary relationships under new changes to the National Institutes of Health’s conflicts of interest policies. NIH told a federal watchdog it will update its regulations and procedures to incorporate non-financial conflicts, according to a report released yesterday. The changes aim to curbing undue foreign influence, which was one of the major issues facing the agency before the pandemic. Jeannie Baumann has more.
More Headlines:
- Care Coordination Pushed for Dual Medicare-Medicaid Enrollees
- Biogen to Pay $22 Million in Drug Kickback Settlement, DOJ Says
- Horizon Sinks 10% as Vaccine Production to Hurt Tepezza Supply
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.