HEALTH CARE BRIEFING: Halt on Care Spurs Record Insurer Profits
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Health insurers enjoyed record profits in the second quarter of 2020 due to the pandemic’s halt on elective surgeries and routine care, credit rating agency AM Best reported.
Net underwriting income in the second quarter increased to $28.3 billion from $9.1 billion in the same prior-year period, AM Best said. Underwriting income is revenue generated by premiums minus the costs of paying claims.
Net income rose to a historically high $26.6 billion in the first half of the year, compared to $24.5 billion in the first half of 2019, the credit rating agency said in a report released yesterday. That increase came despite a $3.1 billion reduction in investment income and a $2.9 billion increase in taxes from the Affordable Care Act‘s health insurer fee in 2020. The fee was waived in 2019.
Health insurers have benefited from the widespread shutdown that began in March as postponed non-Covid care reduced claims costs. That led to a backlash on Capitol Hill. House lawmakers are investigating allegations that insurers are profiting off the pandemic while reducing access to free Covid-19 testing.
UnitedHealth Group yesterday raised its adjusted earnings forecast for 2020 to $16.50 to $16.75 per share, from the prior range of $16.25 to $16.55. Adjusted earnings per share were $3.51 in the third quarter, beating the $3.10 average of analysts’ estimates compiled by Bloomberg. Read more from Sara Hansard.
Hospitals Risk Bankruptcy in Latest Covid Wave: Meanwhile, a grim reality is setting in across the U.S. hospital sector: a surge in coronavirus infections is encroaching while most facilities are still recovering from the onset of the pandemic. The growing number of cases is threatening the very survival of hospitals just when the country needs them most. Hundreds were already in shaky circumstances before the virus remade the world, and the impact of caring for Covid patients has put hundreds more in jeopardy.
The new coronavirus sidelined profitable elective procedures and pushed up costs to keep patients and staff safe. Hospitals are losing the privately insured patients they depend on as millions of Americans lose their jobs and employer-sponsored coverage. “It sort of all comes together as essentially a triple whammy,” Aaron Wesolowski, vice president for policy research, analytics and strategy at the trade group American Hospital Association, said in an interview. Read more from Lauren Coleman-Lochner.
The Coronavirus Pandemic
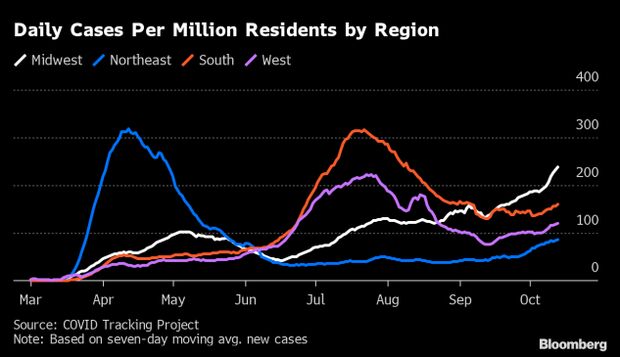
Virus Comeback Spreads Across 46 States: Covid-19’s resurgence has now reached the vast majority of states, with the virus raging in the Midwest and early warning signs flashing just about everywhere else, sending hospitalizations higher. In 46 states and the nation’s capital, the case trend has worsened from a month ago, based on the trailing seven-day average of new infections, according to Covid Tracking Project data. At the end of September, that was true of 32 states; at the end of August, just 15 were trending up. By that metric, cases are dropping only in Georgia, Hawaii, South Carolina and Washington state.
Nationwide, the country added 52,274 cases Tuesday, pushing the seven-day average to 51,027, the highest since Aug. 16. A surge in testing is responsible for part but not all of that increase: The seven-day average of new cases is up 46.5% in the past month; tests are up 28.9%. Current hospitalizations have reached the highest since Aug. 29, according to the Covid Tracking Project. They are rising in all regions. About 216,000 Americans have died from the disease, according to Johns Hopkins University data. Read more from Jonathan Levin.
FDA Chief Says Vaccine-Trial Halts About Safety: A series of trial stops are part of the system to make sure any Covid-19 vaccine or therapy will be safe, FDA Commissioner Stephen Hahn said. “Probably the most important part of clinical trials is to ensure safety as much as possible, certainly for the participants in the trial, but also to look at safety issues should an application come before FDA,” Hahn said in a 30-minute phone interview in which he discussed ongoing vaccine trails, how long it will take the FDA to review an application for a vaccine, public confidence in the agency and drugmakers, and more. Read more from Drew Armstrong.
Hurdles have risen in the race to secure Covid-19 vaccines and treatments, with trials of several protective shots and therapies temporarily halted amid safety concerns, even as other studies advance. With a late-stage test of a vaccine candidate from AstraZeneca and the University of Oxford still on hold in the U.S., Johnson & Johnson paused a trial of its shot, while Eli Lilly temporarily stopped enrollment in a study of its antibody therapy. The moves underline drugmakers’ pledge to uphold scientific rigor in the quest for a way out of the coronavirus crisis, even as assessments of proposed vaccines from the likes of Pfizer and Moderna continue. Read more from Cristin Flanagan, Riley Griffin, Robert Langreth.
Eli Lilly said the potential safety concern that triggered a pause of a high-profile government-sponsored clinical trial of an experimental Covid-19 antibody treatment hasn’t affected its other studies. While that trial, known as ACTIV-3, remains in limbo, the Indianapolis-based drugmaker said yesterday that the independent data safety monitoring board that recommended the pause hasn’t called for enrollment to be paused in another government-run trial it is overseeing, known as ACTIV-2. Read more from Riley Griffin.
In a positive sign for one of the front-runners in the race for a vaccine, the side effects that have emerged in a large late-stage trial of the candidate from Pfizer and BioNTech are in line with those seen in smaller early studies. The partners haven’t had to stop their late-stage study over safety concerns, BioNTech CEO Ugur Sahin said in an interview. They’re sticking with their previously announced target of being able to show as soon as this month whether the vaccine works. That would put Pfizer and BioNTech on track to potentially be the first to show the efficacy of a Covid-19 vaccine. Read more from Naomi Kresge.
States Face Monumental Challenges Readying Vaccine Distribution: As states race the clock to ready their plans to vaccinate millions of people by tomorrow’s federal deadline, they are pushing ahead with the health planning equivalent of wearing blindfolds. State health officials, under intense time pressure, are grappling with a multitude of logistical hurdles, yet they haven’t a clue which vaccine they will be distributing nor when — or even if — a vaccine will be forthcoming.
That’s a problem. Even as President Donald Trump has ceded much of the job of readiness to local leaders for distribution of Covid-19 vaccines, it’s been impossible for states to fully prepare when, for instance, safe storage and transportation requirements will differ vastly depending on which of the four frontrunner vaccines now in contention will be approved. Read more from Angelica LaVito.
More Headlines:
Pandemic Shuts Researchers Out of Labs When They’re Needed Most
YouTube to Pull Clips Questioning Authorities on Covid Vaccines
What Else to Know
Barrett Again Pressed on ACA: Senate Democrats used their second day of questioning to again press Amy Coney Barrett for her thoughts on the Affordable Care Act. During her final day appearing at the confirmation hearings, Barrett continued to avoid answering direct questions about how she would vote in potential Supreme Court cases. She said she wasn’t calling for the court to be more aggressive in overturning its precedents. She repeated that she had no “hostility” toward the Affordable Care Act, which the court will consider overturning in a Nov. 10 argument.
“No matter what somebody’s policy preferences are about the ACA, I completely agree with you they shouldn’t be trying to undermine the policy that Congress enacted,” Barrett said in response to Sen. Dick Durbin (D-Ill.). Durbin said Trump had placed a “cloud over your nomination” by declaring his intention to add to the Supreme Court a justice who would vote to overturn that law.
Sen. Amy Klobuchar (D-Minn.) intimated that Barrett took a stance against the ACA with an eye toward getting a judicial appointment from Trump. Klobuchar pressed Barrett to say she was aware of Trump’s opposition to the law when the then-professor wrote a 2017 law review article that criticized Chief Justice John Roberts’s opinion upholding the measure. Not long after it was published, Trump nominated Barrett to a federal appellate court and she was confirmed. Barrett said she probably wrote the article before the 2016 presidential election. “To the extent you are suggesting that this was like an open letter to President Trump, it was not,” she said. Read more from Greg Stohr and Laura Litvan.
More Headlines:
Heart, Lung Rehab Via Telehealth Covered Under Medicare
Tennessee’s 48-Hour Waiting Period for Abortions Struck Down
Purdue’s Handover of Opioid Maker Should Be Nixed, State AGs Say
Overdose Deaths Reached Record High in Months Before Pandemic
Appeals Court Urged to Uphold Order Vacating HHS Conscience Rule
To contact the reporter on this story: Zachary Sherwood in Washington at zsherwood@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.