HEALTH CARE BRIEFING: Faulty, Incomplete Data Harms Covid Fight
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
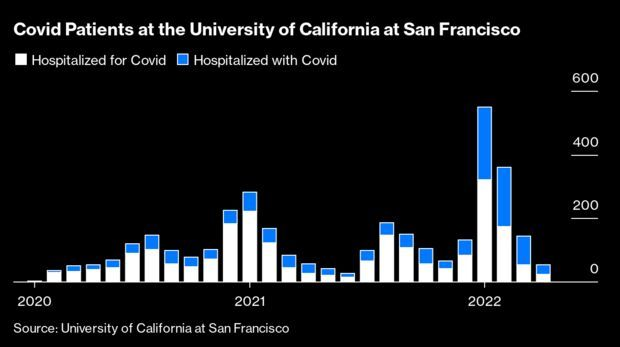
In January, Massachusetts added a new set of figures to its Covid dashboard. Two years into the pandemic, it began to draw a distinction between people who were hospitalized because of the virus and people who were there for other reasons but also happened to be infected. Nothing changed inside the hospitals’ walls—a patient with Covid there due to a car crash still had to be isolated. But the effect on the state’s numbers was dramatic—it cut them in half.
While cases have plunged and the death tally is slowing, the U.S. will in the next few weeks pass 1 million Covid fatalities, and it’s likely that more than half the country has been infected. There will almost certainly be more waves of illness—either from new variants or as a seasonal event. When those waves come, they will hopefully be less deadly, thanks to a wall of immunity from vaccinations and prior infections. And thanks to that, political leaders, health experts, and regular Americans across the country are adopting new attitudes toward risk and what costs they’re willing to pay to stop transmission.
But they’re making those choices with flawed, or at least outdated, information, due in part to the U.S.’s fractured public health system. It’s a deficit that has made it harder to assess the consequences of the pandemic and what we’re willing to do to avoid those costs, and has helped create a vacuum that’s been filled by fatigue and distrust.
Even after billions of dollars in spending and a million dead, the way Americans measure the risk of the virus hasn’t improved much in the past two years. The question of how many people are hospitalized is key—new thresholds for public health rules from the Centers for Disease Control and Prevention depend on it. If the virus does return in another wave, it’ll be essential to know how much vulnerability exists in communities—but U.S. data systems make that difficult. And how can Americans spot a wave when more people either stop testing or shift to at-home tests that don’t get reported?
Through the pandemic, tallying hospitalizations has been one of the best ways of measuring the virus’s consequences. Case numbers undercount the number of sick and lump in the barely symptomatic with the gravely ill. Deaths are a final reckoning but come weeks or even months too late to have any predictive value. Hospitalizations tally the stresses on the health system and the financial costs, as well as the impact on those who spend weeks in an inpatient bed.
In the first year and a half of the pandemic, hospitalizations were a simple measure: Almost everybody infected with the virus who ended up in the hospital during the surges was there because of the virus. But during the wave of omicron-variant driven disease that started late last year, something changed: About half the people with Covid who entered the hospital were there for something else. Drew Armstrong has more.
Booster Rule Changes May Widen Inequities: Ever-changing guidance on Covid boosters could widen disparities in uptake for low-income and minority groups that tend to face barriers to health information. Some advisers to the Centers for Disease Control and Prevention said most Americans under 50 should wait for the next generation of boosters rather than get a fourth dose now to prevent Covid-19 infections. CDC Officer Sara Oliver said at a meeting last week that because second boosters are already available for certain groups, the agency “can rapidly adjust recommendations” if the epidemiology of Covid changes.
But public health policy officials warn that repeatedly altering the guidelines will cause confusion and stir hesitancy among those with limited opportunities to get their questions answered from trusted health-care providers. It “will initially result in profound equity issues,” Matthew Zahn, deputy health officer for the Orange County Health Care Agency, said at the agency meeting. “The communities that are at highest risk will initially be those with the lowest immunization rates.” Read more from Celine Castronuovo.
U.S. Moves to Widen Use of Pfizer Covid Pill as Supply Grows: The Biden administration will allow all pharmacies to order Pfizer Inc.’s Covid-19 therapy pill, as it looks to boost access to the promising drug as the supply increases. The administration announced new steps on Tuesday to expand access to the Paxlovid pills and encourage broader use, senior administration officials said, speaking on condition of anonymity to discuss the plan.
Starting this week, tens of thousands of pharmacies will be able to order the drugs, and the administration will launch more sites where people can be tested, examined and get pills in one stop — a so-called test-to-treat system that’s been slow to get underway since Biden announced it in the State of the Union. Read more from Josh Wingrove and Riley Griffin.
Hospitals Struggle to Absorb Rising Pandemic Costs: U.S. hospitals are struggling to absorb rising costs for labor, drugs and supplies as the pandemic drags on, the American Hospital Association said Monday in a report. Labor costs per patient jumped by 19% in 2021 from 2019, and supplies rose by over 20% per patient in that period, according to the report. Nursing expenses shifted heavily toward travel nurses. The travelers’ share of nursing budgets rose to 39% in 2022 from 5% in 2019. The U.S. has allotted over $170 billion to help hospitals in the pandemic, but many say they’re still losing money. Carey Goldberg has more.
More Coronavirus Coverage:
- FDA Widens Gilead’s Veklury Approval for Covid Treatment
- Retail Pick Veru Jumps as Covid Drug Cuts Deaths in Study
- Sorrento Gets FDA Green Light for Phase 1 Covishield Trial
- Covid Vaccine Order for California Prison Workers Gets Tossed
Also on Lawmakers’ Radar
FDA User Fee Agreements: The Senate Health, Education, Labor, and Pensions Committee holds another hearing on user fee agreements between the Food and Drug Administration and the medical industry, which are being negotiated ahead of legislation that would enshrine them into law. Today’s session includes three directors of FDA centers. For more on the authorization legislation, see the BGOV OnPoint: Congress Ramps Up for FDA User Fee Reauthorization.
Opioid Probe Eyes Risk Process Led by Fed’s Barkin: A congressional investigation into global consultancy McKinsey’s role in advising its client on promoting opioid sales includes a look at “risk-management” processes that were overseen for a time by the current president of the Federal Reserve Bank of Richmond. Thomas Barkin, who was chief risk officer at McKinsey before joining the Richmond Fed, hasn’t been a direct focus of the House Oversight Committee’s investigation. But an interim report shows lawmakers are delving into the firm’s oversight of its engagement with Purdue Pharma, makers of OxyContin.
The committee will hold a public hearing into the consultancy firm’s role in the opioid epidemic on Wednesday in Washington. McKinsey has so far not fully cooperated with lawmakers, according to the report, which stated the firm “failed to provide core documents concerning the identity of its private sector clients and details of complaints or concerns raised to McKinsey’s risk-management committees.” Read more from Billy House, Christopher Condon, and Craig Torres.
Lobbying Effort to Lower Drug Prices Launches: Long-time supporters of legislation to allow the government to demand drugmakers lower their prices will announce today the launch of a new campaign to reenergize Democrats’ stalled effort to pass a sweeping domestic agenda before the end of May.
AARP, SEIU, the Purchaser Business Group on Health, Patients for Affordable Drugs Now and dozens of other advocacy organizations will host an event with Sen. Amy Klobuchar (D-Minn.) unveiling plans for persuading the Senate to advance the same drug pricing legislation that passed the House in 2021.
Several of the groups already spend millions of dollars each year advocating on public policy issues, including drug pricing.
The House-passed bill (H.R. 5376) was effectively killed by opposition from Sen. Joe Manchin (D-W.Va.), who has said he supports the drug pricing provisions of the legislation but not other spending items in it. The package has no Republican supporters, so it needs backing of all 50 Senate Democrats for passage, Alex Ruoff reports.
What Else to Know
Anthem Prosthetics Settlement Wins ‘Conditional’ Approval: A class settlement requiring Anthem to expand its coverage of computerized knee and foot-ankle prosthetic devices won “conditional” approval from a California federal judge, who warned that more information must be filed before the deal can be finalized. Read more from Jacklyn Wille.
Delayed Kidney Cancer Diagnosis Suit Ditched by Iowa Court: The estate of a woman, who died of metastatic kidney cancer years after an emergency room doctor failed to tell her or her family physician about a cyst in a CT scan, sued the doctor too late, the Iowa Supreme Court said. Read more from Mary Anne Pazanowski.
More Headlines:
- ‘Pharma Bro’ Shkreli Fails to Lift Industry Ban During Appeal
- UnitedHealth Subsidiary Cites DaVita Loss in No-Poach Lawsuit
- Blue Cross Reimbursement Case Revived in Win for Indian Tribe
- Axsome Shares Tumble as CRL Expected for Migraine Medicine
- AstraZeneca, Alembic Settle Patent Suit on Cancer Drug Tagrisso
- Otsuka Sues Teva to Block Copies of Jynarque Kidney Disease Drug
- Health-Care Company IQVIA to Pay $550,000 to Settle Bias Suit
With assistance from Alex Ruoff
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.