HEALTH CARE BRIEFING: Democrats Tack Health Agenda Onto Stimulus
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Democrats are betting they can open the door for major health changes by first taking on modest, temporary expansions of insurance assistance and public coverage programs.
House Democratic committee leaders are preparing an estimated $1.9 trillion relief measure, which includes two years of increased subsidies for people buying insurance on Obamacare’s individual markets. It also carries temporary support for people attempting to keep employer-sponsored insurance and added funds to pressure states to expand their Medicaid programs.
A dozen states haven’t expanded Medicaid yet, including Florida and Texas, two of the most-populous states.
These changes—most of them included in the House Ways and Means portion of the package—are short of the promises that Democratic leaders have made to lower health-care costs for most Americans and to extend coverage to millions more Americans. But Democrats and their allies say the relief package moves them closer to making good on those promises.
“It’s a critical step forward to build on the Affordable Care Act,” according to Leslie Dach, chair of Protect Our Care, a group allied with Democrats that’s spent years advocating for the ACA. “History has shown that when people experience the benefits of the ACA,” he said, “they will will fight to keep it.”
The aid package is an “important down payment” on President Joe Biden’s vows to lower cost-sharing for people with insurance and to tackle drug prices, said Chris Jennings, a Democratic consultant who worked on Biden’s transition, Alex Ruoff and Sara Hansard report.
Energy and Commerce Stimulus Markup: The House Energy and Commerce Committee’s stimulus proposal would make big changes to Medicaid, Alex Ruoff and Erik Wasson report. The committee plans to mark up the legislation today at 11 a.m. It also offers states the ability to claw back money from price increases of certain drugs.
Biden Faces Pandemic Without Key Health Officials
President Joe Biden is fighting his war against the coronavirus shorthanded, as delays in the Senate and by his own White House have left several top U.S. health posts unfilled.
Biden’s team lacks a confirmed Health and Human Services secretary and surgeon general. And the president has yet to name permanent heads for the Food and Drug Administration, which approves vaccines, or the Centers for Medicare and Medicaid Services, which administers the government’s two large health programs for elderly, disabled and low-income people. Other roles are also unfilled.
The holes threaten to hamper the U.S.’s rebound from the virus and undermine one of the biggest promises of Biden’s campaign. The logjam rests in large part in the Senate, which has found scant time to confirm Biden’s key nominees as lawmakers haggled over how to split power and are now contending with the impeachment trial of former President Donald Trump.
The president isn’t likely to see immediate relief. Xavier Becerra, Biden’s pick for health secretary, won’t even have Senate committee hearings until the week of Feb. 22, though the planning remains fluid, aides familiar with the matter said. That would probably push any full confirmation vote into March.
The White House is stepping up the pressure. “Frankly, it is disappointing that Congress, the Senate, is delaying any further in confirming his nomination at a time when thousands of people are dying every day of a pandemic and people need leadership at the top of an agency that has an important role to play,” Press Secretary Jen Psaki said yesterday. Read more from Josh Wingrove.
- House Appropriations Chair Rosa DeLauro (D-Conn.) sent a letter asking Biden and Becerra to rethink how the U.S. addresses migrant children at the border to “avoid placing children unnecessarily in the Unaccompanied Children program and to explore other avenues for placing children in shelters before expanding an influx shelter.” DeLauro is also chair of the Labor-HHS-Education Subcommittee. Read her letter here.
Biden Justice Department Flips Trump Stance on ACA: Biden’s administration told the U.S. Supreme Court the Affordable Care Act is constitutional, filing an unusual letter that flips the government’s position three months after the justices heard arguments on the law. Trump’s administration had argued against the health-care law, also known as Obamacare, when the justices heard the case Nov. 10. Opponents are trying to invalidate the entire law by pointing to a 2017 tax change that eliminated a penalty for not having insurance. Greg Stohr has more.
ACA Architect Could Lead Value-Based Care Office: Elizabeth Fowler, who led the drafting of the Affordable Care Act and then implemented the major health law, has emerged as the top contender to run the Health and Human Services Department’s Center for Medicare and Medicaid Innovation, according to people familiar with the matter. Shira Stein has more.
Americans Lacking Coverage Fell Early in Pandemic: Almost 3 million fewer people lacked health insurance in the first six months of 2020 than in 2019 despite millions of people losing their jobs amid the pandemic, the Centers for Disease Control and Prevention said yesterday. From January through June 2020, 30.4 million of all ages—9.4% of the U.S. population—lacked coverage, slightly lower than the 33.2 million people (10.3%) who were uninsured in 2019, the CDC said. Read more from Sara Hansard.
The Coronavirus Pandemic
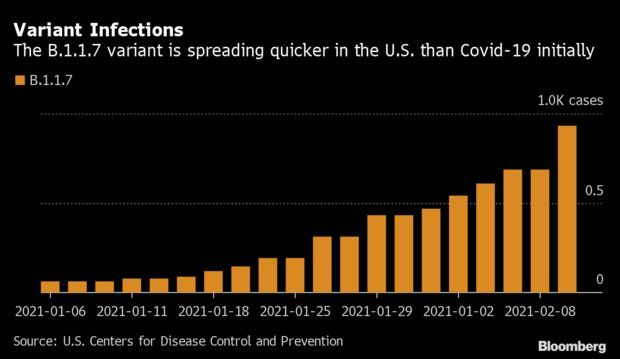
U.K. Variant Gains Momentum in U.S.: A coronavirus strain first found in the U.K. is infecting Americans at a pace that far outstrips the original strain, which arrived in the spring. In the 43 days since B.1.1.7 was first found in the U.S. over 900 cases have been reported. At around the same point, the first strain had infected only 165 people, although testing capacity at the time was weaker. Even as overall daily cases decline, the B.1.1.7 mutation is burgeoning faster than others first seen in Brazil and South Africa. Read more from Nic Querolo.
- The daily number of overall new coronavirus cases in the U.S. was under 100,000 for a third consecutive day Tuesday, the first time that’s happened since the week of Nov. 2, according to data compiled by Johns Hopkins University and Bloomberg. On Jan. 5, the U.S. posted a record 405,982 new infections after a holiday season when many Americans decided to travel. Read more from Bloomberg News.
CDC Study Says Double-Masking Could Slow Spread: Wearing a cloth mask over a medical mask can boost protection from aerosolized particles and slow the spread of Covid-19, a new study from the U.S. Centers for Disease Control and Prevention found. The study, part of the agency’s Morbidity and Mortality Weekly Report, also examined the efficacy of modifications made to improve the fit of a medical mask. Read more from Emma Court.
Astra Shot Backed by WHO for All Adults: A World Health Organization panel recommended AstraZeneca’s Covid-19 vaccine for all adults over 18, paving the way to speed up inoculations in developing countries. The recommendation may encourage more countries to use the shot broadly, after some European Union members advised against giving it to the elderly because of insufficient trial data. Read more from Corinne Gretler.
One Dose of Pfizer Shot Has 65% Efficacy, Study Shows: One dose of the Pfizer-BioNTech vaccine offers two-thirds protection against coronavirus, data seen by the U.K. government suggests. Early findings from the U.K.’s vaccination program due to be released within days show that the first dose reduced the symptomatic infection risks among patients by 65% in younger adults and 64% in over-80s, a person familiar with the matter said. Read more from Tim Ross and Emily Ashton.
Justices Ideological in Covid Rulings, New Data Project Says: Supreme Court justices have drawn “obvious battle lines” in Covid-19-related cases, according to Adam Feldman, founder of The Juris Lab, a new forum for data-driven legal analysis. “The conservative position seeks more rights for prayer and fewer exceptions made in voting and prison related cases on the basis of impediments stemming from the possible spread of COVID,” Feldman said yesterday in blog post at the site, which launched this week. Read more from Jordan S. Rubin.
More Headlines:
- CVS Looks to Simplify Vaccine Site for Federal Pharmacy Push
- BioNTech Supercharges a Factory to Produce More Covid Vaccine
- Airlines Opposed to Pre-Flight Tests to Meet With Biden’s Virus Czar
- Nursing Homes Want More CDC Data on Covid-19 Shot’s Effectiveness
- U.S. to Open Mass Vaccine Sites for Vulnerable New York Residents
- AstraZeneca, IDT Biologika Weigh Options to Boost Vaccine Output
- Newsom Touts Vaccine Gains, as California Deaths Near U.S Record
What Else to Know Today
Trump EPA Actions Caused Deaths, Lancet Finds: The Trump administration deliberately harnessed racism and class animosity to push policies that caused hundreds of thousands of U.S. deaths, according to a scathing new report in the British medical journal The Lancet. After undertaking a comprehensive assessment of the health and environment impacts of Trump’s presidency, the 33 scientists who co-authored the article estimated that rollbacks of environmental and workplace protections led to 22,000 excess deaths in 2019 alone. They also found that 40% of U.S. deaths during 2020 from Covid-19 would have been avoided if the country’s death rate had been closer to that of its G7 peers, and blamed Trump for eschewing the advice of public health agencies and politicizing common sense responses to the pandemic such as mask-wearing. Read more from Leslie Kaufman.
Immigration ‘Wealth Test’ Review Halts Legal Challenge: A lawsuit challenging the Trump administration rule withholding legal status to most immigrants who receive public assistance was suspended pending review of the rule ordered by Biden. The “public charge” rule sought to refuse green cards to any immigrants who used Medicaid, food stamps, housing vouchers or other forms of government assistance. Read more from Bob Van Voris.
Teva Patent Case Redo Spotlights Use of Generic ‘Skinny Labels’: A Federal Circuit rehearing in a patent case against Teva Pharmaceutical Industries could offer clarity over the extent to which generic drugmakers can rely on a once surefire practice for quick market entry. At the center of the debate is the use of “skinny labels,” which generic companies depend on to sell copycat versions of a branded medicine, but only for limited uses. The initial appeals court ruling threw into question the viability of that practice, which has provided a gateway for many drugmakers to enter the market without infringing a brand owner’s patent for a particular indication. Read more from Ian Lopez.
More Headlines:
- VA Wants Support for Electronic Health Records Implementation
- Nevada Health System Owes $75,000 in HHS Records Settlement
- AbbVie’s Allergan Says Botox Gets FDA OK for Pediatric Detrusor
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.