HEALTH CARE BRIEFING: CDC Advisers Punt on J&J Vaccine Decision
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
U.S. public health advisers concluded a meeting on Johnson & Johnson’s coronavirus vaccine without a vote, effectively extending a halt on its use as they seek more data on an extremely rare blood clotting side effect.
After scrutinizing evidence of the clots during an hours-long emergency meeting, advisers to the U.S. Centers for Disease Control and Prevention said they lacked adequate information to make recommendations on how to respond to reports of the clots. The Advisory Committee on Immunization Practices didn’t give a precise date on when they’ll reconvene to reconsider the vaccine but said it could be in a week to 10 days.
After six women who received the J&J vaccine developed a severe form of blood clotting, the CDC and the Food and Drug Administration jointly recommended the pause on its use earlier this week. Members of the panel, who are independent of the agency, considered data from those cases alongside similar incidents tied to AstraZeneca’s coronavirus vaccine in Europe.
“I want to be able to feel comfortable with my family members and with myself for that matter to receive this vaccine,” said Beth Bell, a member of the CDC advisory panel and professor at the University of Washington.
They weighed concerns over J&J’s shot with risks from contracting Covid-19, which has killed 564,098 people in the U.S., according to the latest count from Johns Hopkins University. The stakes are high, with variants spreading, especially in the Midwest, and threatening to erase the gains the U.S. has made against the virus with vaccination.
Some panel members advocated for a monthlong pause. Sarah Long, a member of the panel and professor at Drexel University, said she backs a pause of that duration and that she’d be opposed to resuming use of J&J’s vaccine at this point in time. Advisers also discussed potentially restricting Johnson & Johnson vaccine access to certain age groups, as Europe did with AstraZeneca’s vaccine.
Others were concerned about the effects of not having the J&J vaccine available, especially to the communities it was being targeted toward such as hard-to-reach communities. One member noted that what ACIP chooses to do could have global implications, with many poorer countries expecting to receive the J&J vaccine, Joe Schneider reports. Read more from Angelica LaVito.
- The U.S. vaccination drive has failed to equitably reach people of all races, and the pause on the Johnson & Johnson Covid-19 vaccine is halting key efforts to jab hard-to-reach minority populations. The J&J shot only makes up about 4% of the more than 189 million doses administered. But because it doesn’t require expensive refrigeration and is only one dose, it’s the vaccine of choice for programs aimed at inoculating people who are homebound, homeless, and whose jobs make it hard to schedule multiple appointments. These are groups disproportionately made up of the minority populations already lagging in doses. Read more from Jeff Green.
Liability Shield a Boon Despite Clots: Johnson & Johnson, AstraZeneca, and other makers of Covid-19 vaccines are shielded from liability for adverse effects on recipients, and lawyers say the protections are a boon for medical innovation critical to battling the pandemic. Roughly 7 million Americans have received the J&J vaccine, which makes the severe blood clotting cases extremely rare. But the incidents highlight how drugmaker protections are key for permitting fast-paced clinical trials and output when speed is key to an effective national response. Read more from Ian Lopez and Jacquie Lee.
Happening on the Hill
House GOP Seeks to Force Abortion Bill Vote: House Republican leaders say they’re hoping to force a vote on a “born alive” antiabortion bill that threatens doctors with criminal and civil action if they don’t provide medical care to a child that survives an abortion. But their move is largely symbolic; infanticide is already illegal and abortion-related complications occur in less than 1% of procedures. Still, the measure is backed by influential antiabortion groups.
House Minority Whip Steve Scalise (R-La.) said he hopes Republicans can gather the required 218 signatures needed to complete the discharge petition and force a chamber vote, bypassing the committee process. Scalise said he needs five Democrats to back a united GOP caucus. “You can’t be pro-life and you don’t sign that discharge petition,“ he said.
One problem for Republicans: there aren’t five Democrats who regularly support antiabortion bills in the chamber. Rep. Henry Cuellar (D-Texas) is the only House Democrat still in office who’s joined Republicans in the past supporting the Trump administration’s restrictions on family planning funds. The Democrats who joined Republicans in supporting the last “born live” discharge petition, ex-Reps. Collin Peterson (D-Minn.), Ben McAdams (D-Utah) and Daniel Lipinski (D-Ill.), lost re-election last year, Alex Ruoff and Emily Wilkins report.
House Clears Biosimilars, Chemical Entity Legislation: The House passed a bill yesterday aimed at lowering drug prices (S. 164) that would direct the Department of Health and Human Services to create a website for educational materials on biosimilar medicine. The Senate passed the bill earlier this year and it now heads to Biden for his signature, Alex Ruoff reports. Read the BGOV Bill Summary by Brittney Washington.
- Another drug pricing measure (S. 415) passed the House by voice vote yesterday. That bill would adjust the criteria for a drug to receive exclusivity as a new chemical entity, narrowing which medicines get a five-year monopoly. It would help lower drug costs by increasing competition and closing loopholes that prevent generics from coming to market, Ruoff reports. “We must lower prescription drug costs,” Sen. Bill Cassidy (R-La.), the bill’s sponsor, said in a news release. “We can do this by increasing competition and closing loopholes which prevent generics from reaching the marketplace. This bill does both.” The legislation passed the Senate earlier this year. Read the BGOV Bill Summary by Danielle Parnass.
Cornyn Calls for Probe of Avantor Link to Drug Crisis: Sen. John Cornyn (R-Texas) formally requested that the Justice Department and the Securities and Exchange Commission probe Avantor for what he called the company’s “deeply troubling connection” to the opioids crisis, citing its “apparent longstanding contribution to the opioid epidemic that killed 50,000 of our fellow citizens in 2019.” Cornyn, who sent a letter to Attorney General Merrick Garland, has served for over a decade on the Senate’s Caucus on International Narcotics Control. Read more from Cam Simpson.
- Although his letter makes no mention of it, Cornyn’s requests come amid a partisan Senate fight over one of Biden’s Justice Department nominees who has financial ties to Avantor. Vanita Gupta, Biden’s choice to be associate attorney general, has held a $14.5 million stake in the company that she pledged to sell following questions from Republicans. The Senate is scheduled today to vote on a motion to proceed in order to discharge Gupta’s nomination from the Judiciary Committee.
Loosen Telehealth Restrictions, Employers to Say at Hearing: Congress should allow medical providers to offer telehealth services across state lines and allow those services to be covered without requiring that annual deductibles be met, a group representing large employers plans to tell a congressional subcommittee today.
“Employers have seen an increasing need for mental and behavioral health care—including opioid addiction— among employees and their families, especially with the arrival of COVID-19,” and they have developed new programs to increase access to treatment, James Gelfand, senior vice president of health policy for the ERISA Industry Committee (ERIC), planned to say in prepared testimony to a subcommittee of the House Committee on Education and Labor. ERIC represents large employers that provide health, retirement, and other benefits. Read more from Sara Hansard.
- The House Education and Labor Subcommittee on Health, Employment, Labor, and Pensions will livestream its hearing on access to behavioral and mental health care here.
More Hearings Today:
- Nominations: The Senate Finance Committee holds a hearing on the nominations of Andrea Joan Palm to be a deputy secretary, and Chiquita Brooks-LaSure to be administrator of the Centers for Medicare and Medicaid Service, both of the Health and Human Services Department.
- Fauci, Walensky Testify on Virus: The House Select Subcommittee on the Coronavirus Crisis plans a hearing on a science-driven approach to ending the Covid-19 pandemic. Witnesses include CDC Director Rochelle Walensky, National Institute of Allergy and Infectious Diseases Director Anthony Fauci, and Health and Human Services Department Covid Response Chief Science Officer David Kessler.
- Trusted Vaccine Information: The Senate Commerce, Science and Transportation Subcommittee on Communication, Media, and Broadband meets for a hearing on communicating trusted vaccine information. National Association of Broadcasters President Gordon Smith and others will testify.
- Fiscal 2022 Appropriations: The House Appropriations Labor-HHS-Education Subcommittee will review the Health and Human Services Department. HHS Secretary Xavier Becerra will testify.
Spy Agencies Still Investigating Virus Source: U.S. spy agencies also have yet to determine the source of the virus that causes Covid-19, Director of National Intelligence Avril Haines told the Senate Intelligence Committee at a hearing on worldwide threats yesterday. She said analysts are still examining two theories: animal-to-human transmission, and the possibility of a laboratory accident. Burns added that “the Chinese leadership has not been fully forthcoming or fully transparent” in working with the WHO to pinpoint the origin of the coronavirus. Read more.
More on the Pandemic
Tai Urges WTO to Consider Changes for Virus Fight: U.S. Trade Representative Katherine Tai, in testimony at a World Trade Organization meeting, said inequality in Covid-19 vaccine access among nations is unacceptable and asked countries to consider whether changes to the WTO’s rules may be needed to avoid a situation that leads to more deaths. She said the world must not repeat mistakes of the past, in which various policies and actions regarding the HIV/AIDS epidemic constrained access to medications, Eric Martin reports.
Airlines’ Middle Seats Cited as Risky: The risk of being exposed to the Covid-19 virus on an airline flight drop by up to half when airlines keep middle seats open, a new study published by the U.S. government concludes, a safety practice the carriers have abandoned. The report is the latest to roil the waters on a controversial topic: just how dangerous it is to travel amid the pandemic. The risk of coming into contact with the virus fell by 23% to 57% if airlines limit passenger loads on single-aisle and widebody jets, compared to full occupancy, according to research. Read more from Alan Levin and Mary Schlangenstein.
Most Approve of Biden, Vaccine, New Poll Shows: Most Americans approve of the job Biden is doing, in particular on dealing with the coronavirus, but still one in five are firm in their refusal to get a vaccine, according to a poll. A Monmouth University poll released yesterday showed Biden’s overall approval rating has risen to 54% from 51% one month ago, with 62% of those surveyed saying he is doing a good job tackling the virus. But 21% of Americans state they will never get a vaccine to prevent infection if they can avoid it. Read more from Emma Kinery.
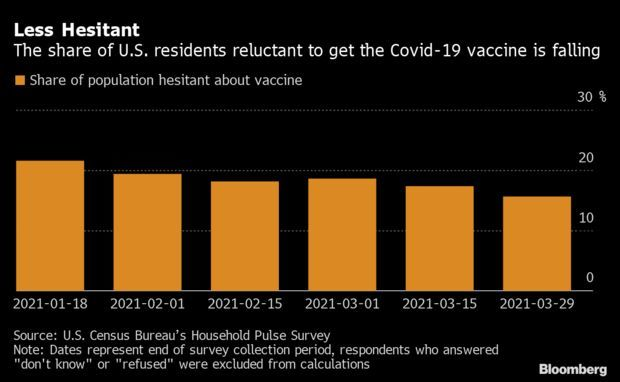
- A separate poll found that 1 in 7 Americans remain wary about getting a Covid-19 shot, largely because of concerns about their side effects. That segment is younger and less educated than average, according to a tracker released by the U.S. Census Bureau that uses Household Pulse Survey data. Residents were surveyed before the U.S. yesterday recommended pausing Johnson & Johnson vaccinations. Read more from Nic Querolo.
More U.S. Headlines:
- NIH Closes Enrollment for Trial Comparing Covid-19 Treatments
- Patent Office Begins Fast-Track for Covid-19 Application Appeals
- Novavax, Florida Cancer Center Build Legal Teams After Scrutiny
- Shot Injecters at Atlantic City Convention Hall Race Against Time
- Efforts to Increase Vaccine Availability and Perspectives on Initial Implementation (GAO)
Pfizer Boosts EU Vaccine Supply: Pfizer and BioNTech will raise Covid-19 vaccine deliveries to the European Union by 25% this quarter, helping the EU overcome delays to the vaccine from Johnson & Johnson as the drug regulator speeds up its safety review. The U.S. drugmaker and its partner will bring forward 50 million deliveries scheduled for the fourth quarter, adding to the 200 million already planned. The company provided 66 million in the first three months of the year. Read more from Naomi Kresge and Viktoria Dendrinou.
More Global Headlines:
- Missed Opportunity Saw China Fall Behind on Covid-19 Vaccines
- South Africa Accuses J&J of Unreasonable Demands on Vaccines
- USAID, CDC Assessment on Infectious Disease Threats before Coronavirus (GAO)
What Else to Know Today
HHS Proposes Reversing Abortion Referral Rule: A Trump-era rule that prohibited federally funded doctors from referring pregnant patients to facilities that provide abortions would be reversed under a proposal offered by the Health and Human Services Department yesterday. The plan would also undo requirements for “strict physical and financial separation” between clinics that receive federal funds and facilities that provide abortion services. Read more from Fawn Johnson and Lydia Wheeler.
- Also this week, a doctors’ group and the government jointly asked a federal court to halt litigation over a restriction that kept women from accessing a drug to induce abortions, as the Food and Drug Administration suspended the provision. The American College of Obstetricians and Gynecologists sued the FDA to enjoin enforcement of a rule requiring in-person dispensing of mifepristone for the duration of the pandemic. Requiring women to go to a hospital, clinic, or doctor’s office to get the drug unnecessarily exposes them to the coronavirus, ACOG said. Read more from Mary Anne Pazanowski.
Employers Must Tell Ousted Staff of Health Subsidies: Employers have little time to waste in notifying laid-off employees who qualify about access to free health-care coverage under the American Rescue Plan signed into law last month by Biden. The law provides fully subsidized coverage under the Consolidated Omnibus Budget Reconciliation Act (COBRA) from April 1 to Sept. 30 for employees who were involuntarily laid off or lost their job-based health-care plans due to reduced hours during the Covid-19 pandemic. Read more from Sara Hansard.
With assistance from Emily Wilkins
To contact the reporters on this story: Brandon Lee in Washington at blee@bgov.com; Alex Ruoff in Washington at aruoff@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.