HEALTH CARE BRIEFING: Califf to Face Questions on Industry Ties
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President Joe Biden’s pick to lead the FDA will face questions on his longstanding ties to the pharmaceutical industry and the agency’s role in combating the Covid-19 pandemic at a key confirmation hearing tomorrow.
Robert Califf, who previously led the Food and Drug Administration in the final year of the Obama administration, will detail his qualifications for the position at a Senate Health, Education, Labor, and Pensions Committee hearing—a crucial step in his path toward reassuming the reins of the agency.
Former FDA officials say widespread respect for Califf within the agency and across the drug industry can help push him across the finish line. But opposition from some Democratic lawmakers, who say the FDA should play a stronger role in addressing the opioid crisis and lowering drug costs, argue that Califf’s industry connections could get in the way.
Another issue for the committee is how Califf could help restore trust to the FDA, which has faced scrutiny over whether political pressure has influenced the Covid-19 vaccine and booster shot authorization process. Some questions that’ll be directed toward Califf tomorrow “are meant to actually get information from the nominee,” while “some of the questions are for senators to just make a point,” Howard Sklamberg, who previously served as deputy commissioner under Califf, said.
Other comments “are really for a senator to stake out an interest in an issue that the senator hopes or wants the commissioner to take up when the commissioner is in office,” he added.
Califf will also likely face questions on integrating real-world evidence into clinical trials, an issue in which he took interest during his previous run as FDA commissioner. Policy analysts say that resuming domestic and foreign inspections on medical products, which were largely halted amid the pandemic, may also be an area of concern for some senators. Read more from Alex Ruoff and Celine Castronuovo.
Also Happening on the Hill
Biden’s Economic Package Risks Languishing: Senate Majority Leader Chuck Schumer (D-N.Y.) insists the Senate will pass President Joe Biden’s nearly $2 trillion tax-and-spending package before Christmas, but there’s still much work to do and time is running short. A delay into the new year risks slowing momentum for Democrats who need this legislative victory behind them as they fight to maintain narrow majorities in the House and Senate in the 2022 midterm elections. The signature bill includes spending on Democratic priorities such as child care and climate change and drastically changes the tax cuts Republicans won under President Donald Trump.
“There is no way they’re going to be ready to vote on their big bill,” Sen. John Thune (R-S.D.), the No. 2 GOP leader, said on Thursday. Democrats “probably need to nip that fairly soon because it’s just not practically going to happen,” he added. Key pieces are still under negotiation, including a House-passed provision for four weeks of paid family and medical leave that moderate Sen. Joe Manchin (D-W.Va.) opposes. His vote is crucial for passage without any Republican support. Read more from Laura Litvan and Laura Davison.
Increased Obamacare Subsidies in New Senate Bill: Increased funding for labor enforcement, job training and health care are all part of the updated proposal published on Saturday by a Senate panel as part of a massive tax and spending bill that could see floor action in the next few weeks. The updated text from the Senate Health, Education, Labor, and Pensions Committee largely mirrors language the House passed last month. It’s possible the measure will need to change before it hits the Senate floor, but the main components—including expanded access to health care—are likely to stay in place.
The Senate Finance Committee also released a draft of its plan with a series of tax provisions. That measure carries a provision opposed by Manchin to establish a new entitlement program giving four weeks of paid family and medical leave to workers who aren’t given the benefit by their employers. It’s unclear whether that provision will remain in the package when the Senate votes.
The Finance Committee’s portion of the package also includes a proposal similar to House-passed language to allow the federal government to negotiate the cost of some of the most costly drugs and insulin, and it would also require drugmakers to provide rebates when they increase prices of drugs under Medicare by more than the rate of inflation.
Meanwhile, the health and labor panel’s proposal includes increased Obamacare subsidies, akin to the House-passed bill, and would for the first time extend them to people who make no more than 138% of the federal poverty level in states where they aren’t currently eligible. It would also set a $35 cap on insulin copays. Read more from Paige Smith.
- The text removes proposed cuts in disproportionate-share hospital payments, which support hospitals providing a large amount of care to Medicaid patients and the uninsured—for which they receive little to no payment. The DSH cuts would have amounted to reductions in payments up to $7.8 billion over 10 years, the American Hospital Association and other hospital groups that lobbied to remove the provision from the bill said, Alex Ruoff reports.
- The package would give low-cost private insurance plans with more tax subsidies to people in the 12 states that have refused to expand their Medicaid programs under the Affordable Care Act. The proposal is meant to help those caught in the “Medicaid gap,” with incomes too high for Medicaid but too low for ACA relief. Supporters of such cuts say that other changes in the package would make it easier for people to afford insurance, reducing the financial pressure on safety-net hospitals and allowing DSH payments to be cut back.
Caucus Chiefs Push for Mandatory Dental Coverage: Top members of the Congressional Hispanic Caucus, Congressional Black Caucus, Congressional Progressive Caucus Chair, and Congressional Asian Pacific American Caucus told Speaker Nancy Pelosi (D-Calif.) and Schumer that comprehensive adult dental care should be a “mandatory component of Medicaid coverage as part of the Build Back Better Act,” while also “maintaining current provisions in the House-passed bill. Find their letter to Democratic leaders here.
Drugmaker Prices Exploited U.S. Market, Probe Finds: Medicare could have saved more than $25 billion if allowed to negotiate better prices for the most costly medicines over a five-year period, according to a new study released by a House committee. The report, the result of a nearly three-year investigation into how pharmaceutical companies set their prices, is meant to underscore the importance of enacting Democrats’ Build Back Better domestic policy package. That measure would direct the U.S. to demand lower prices from drugmakers for expensive drugs.
Drugmakers including Pfizer, Teva, and Celgene specifically target the U.S. for price hikes because there is no government effort to control the price of drugs, according to internal documents released by the House Oversight and Reform Committee. Some of the companies also pumped money into patient assistance programs specifically to boost their revenue, another quirk of the U.S. pharmaceutical marketplace. Alex Ruoff and Celine Castronuovo have more.
House GOP Plans on Backing Challenge to Shot-or-Test Rule: House Republicans are pushing colleagues to sign onto a friend-of-the-court brief opposing the Biden administration’s Covid-19 shot-or-test rule for larger employers, according to a letter seen by Bloomberg Law. The Occupational Safety and Health Administration’s emergency standard has been put on hold since Nov. 6, with a federal appeals court in Cincinnati poised to hear consolidated challenges to the regulation. House GOP Conference Chair Elise Stefanik (R-N.Y.) was among the letter’s authors. Paige Smith has more.
- The U.S. government will appeal a Georgia federal court order that blocked enforcement of Biden’s vaccine mandate for all federal contractors nationwide. Justice Department lawyers filed the notice of appeal, asking for the U.S. Court of Appeals for the 11th Circuit to reconsider the decision and asked for an emergency stay to the order that blocked the mandate. They claimed that not allowing the vaccine mandate to move forward damages the government’s ability to work with companies under terms of its choosing. Erin Mulvaney has more.
Panel Warns Navarro of Subpoena: Former White House trade adviser Peter Navarro was warned he’ll be considered in “willful noncompliance” with a congressional subpoena if he won’t testify on Wednesday to a House committee investigating the Trump administration’s response to the Covid-19 pandemic. Chair James Clyburn (D-S.C.) didn’t specify possible consequences in a letter to Navarro on Saturday. Navarro told the panel in a letter dated Dec. 7 that he won’t testify due to “a direct order from former President Donald Trump.” Read more from Billy House.
- Clyburn’s panel, the House Select Subcommittee on the Coronavirus Crisis, has also scheduled a hearing tomorrow on accelerating Covid-19 vaccinations across the world. The hearing will explore the public health impacts and economic costs of insufficient global vaccination levels, “and what actions are needed to support global vaccination efforts,” according to a statement on the hearing.
SCOTUS Leaves Texas Abortion Providers With Few Remedies
Texas abortion providers have few options for challenging a state law that prohibits the procedure after six weeks of pregnancy following a U.S. Supreme Court decision that left the law in place, while allowing the underlying case to proceed. The law—called S.B. 8—has virtually shut down abortions in Texas and is the most restrictive in the country.
The legal challenge now presumably returns to the U.S. District Court for the Western District of Texas, where it originated. How much the federal trial court can do once it gets there remains unclear. Supreme Court justices said the only valid defendants are state licensing offices like the Texas Medical Board, which the law says can take action against doctors, nurses, pharmacists, and other licensed professionals for alleged violations.
A Texas state court judge’s decision on Dec. 9 raises some possibility that enforcement could be stopped, at least on a county-by-county basis, a reproductive health law expert said. But the law’s ultimate fate won’t be decided any time soon and could end up eclipsed should the Supreme Court rollback abortion rights in a separate case now pending out of Mississippi.
S.B. 8’s enforcement mechanism proved to be a hurdle for plaintiffs who challenged the law in federal court. It’s difficult—if not impossible—to predict who will bring a state-court suit accusing a provider of violating S.B. 8 because citizens are the ones who are deputized to enforce the law. Plaintiffs sued the state’s attorney general and sought orders blocking state-court judges and county courts from accepting filings from private citizens. But the Supreme Court said providers can’t sue them.
Enjoining licensing agencies from taking action against doctors or nurses would eliminate one potential repercussion of an S.B. 8 suit. Providers wouldn’t have to fear losing their licenses for allegedly violating the law, Elizabeth Sepper, a University of Texas at Austin School of Law professor, told Bloomberg Law. But the high court’s verdict otherwise “doesn’t take away any of the threats of S.B. 8 enforcement,” Sepper said. Read more from Mary Anne Pazanowski.
- Abortion-rights advocates said the court effectively blessed Texas’ ban on abortion after six weeks of pregnancy. Marc Hearron, the Center for Reproductive Rights lawyer who argued against the law, said the court “green-lit” the measure and made it impossible for the challengers to get the kind of statewide injunction they need. The verdict could be a bad sign for abortion-rights advocates as they await word on the fate of Roe v. Wade. Read more from Greg Stohr.
- The Supreme Court’s decision also opens the door for copycat laws targeting constitutional rights as long as other states do a little tweaking. The decision will give states “the political cover to go ahead and enact harsh restrictions on abortion providers that” put enforcement in the hands of private parties “through expensive civil lawsuits,” said Ciara Torres-Spelliscy, professor of law at Stetson University. Kimberly Strawbridge Robinson and Lydia Wheeler have more.
- Meanwhile, a Texas state judge ruled that parts of the law are unconstitutional. A provision in S.B. 8 providing “any person” standing to sue any person who allegedly performed or aided an abortion after six weeks’ gestation violates the Texas Constitution’s open courts provision, Judge David Peeples ruled. Also invalid is the new law’s mandate that a court “shall award” a plaintiff no less than $10,000, even if they didn’t suffer any personal harm, he said. Pazanowski has more.
Biden ‘Very Concerned’ by Abortion Verdict: Biden on Friday said he was “very concerned” by the court ruling. “While it is encouraging that the court ruled that part of the providers’ lawsuit may continue, this ruling reinforces that there is so much more work to be done—in Texas, in Mississippi, and in many states around the country—where women’s rights are currently under attack,” Biden said in a statement. Biden also said he would continue to work with Congress on federal abortion rights legislation, though that effort is unlikely to overcome a filibuster in the Senate. Justin Sink has more.
What Else to Know Today
Biden’s 2,700-Item To-Do List Targets Mental Health: Biden released his second regulatory to-do list Friday, detailing almost 2,700 agenda items that define his ambitions to change the environment, transportation, and mental health-care through the federal government’s rulemaking power. Biden officials want to look into how to boost insurance coverage for mental health and substance abuse treatment, White House official Sharon Block said. The list offers a window into how Biden’s administration plans to use federal agencies in the new year to advance his priorities.
The Health Department is moving forward with a final rule that would modify a health privacy law to make it easier for doctors and hospitals to coordinate patient care. Also on the agenda is the Biden administration’s effort to instate a dispute resolution panel to oversee fights within a federal drug discount program that has spurred a slew of challenges across federal courts. The 340B drug discount program helps low-income Americans by requiring drugmakers to provide products to certain health-care entities at lower prices. Read more from Courtney Rozen.
Medicare Panel Eyes Nursing Home Payment Cut: A congressional advisory panel Friday moved to recommend that Medicare payments for nursing homes, home health agencies and inpatient rehabilitation facilities be cut by 5% next year, citing adequate reimbursement rates for such facilities. Only long-term care hospitals would enjoy a payment increase next year under the draft recommendations adopted by the Medicare Payment Advisory Commission on the final day of its December meeting. Read more from Tony Pugh.
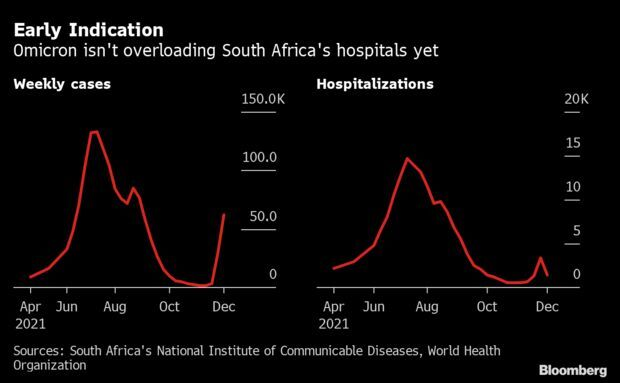
Two Weeks of Data on Omicron: A little more than two weeks since omicron’s discovery a lot has been learned about the latest variant. A lot remains to be discovered. Early data from South Africa, the epicenter so far, shows that the virus appears to spread far faster than previous strains but also doesn’t appear to be causing severe disease. Nothing is definitive yet, so the world is still somewhat in the dark. With omicron cases doubling every few days in the U.K., policymakers are looking at any clues; the spread in Britain could be a harbinger of things to come across Europe and the U.S.
Initial lab analyses indicate omicron is much more transmissible than even delta, the variant that spread rapidly across the globe, filling hospitals and boosting death rates. They also show that it can infect the vaccinated or those who have already been ill with Covid-19. What’s not known yet is how it developed, and whether it will cause more severe disease in countries with older people than South Africa. Also unclear is whether it can out-compete delta in places where that version is dominant now, such as Europe and the U.S. Read more from Antony Sguazzin.
More on the Pandemic:
- Omicron Study Shows Two-Dose Vaccine Induces Fewer Antibodies
- U.K. Says Can’t Rule Out Shutting Schools as Omicron Spreads
- Pandemic Intelligence Chief Brings Data, AI to Variant Fight
- Kentucky Judge Won’t Pause Block on Contractor Shot Rule
- England Could Face 75,000 Covid-19 Deaths This Winter: Study
- Polarized Austria Ends Lockdown as Shot Rule Looms Large
What Else to Know:
- Reboot Ready for VA’s $16 Billion E-Health Data Modernization
- TherapeuticsMD Receives Complete Response Letter From FDA
To contact the reporters on this story: Brandon Lee in Washington at blee@bgov.com; Alex Ruoff in Washington at aruoff@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.