HEALTH CARE BRIEFING: Biden’s Stimulus to Easily Pass House
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President Joe Biden’s $1.9 trillion package will sail through the House when it takes up the bill on Tuesday, according to Democratic lawmakers and aides, even after proposals progressives championed were scaled back.
The Senate’s changes to the House-passed bill, made to appease moderates and comply with parliamentary rules, included dropping a proposed minimum wage increase but are unlikely to prove enough to make progressive Democrats vote against it.
House Speaker Nancy Pelosi (D-Calif.) can only afford to have four Democrats oppose a bill for it to pass, if all members are voting and Republicans are lined up in opposition, given her party’s thin majority. Two Democrats joined a united GOP in voting against the initial version of the stimulus.
Pelosi on Sunday predicted the bill will pass.
“In two days, the House will have a resounding, hopefully bipartisan, vote for justice,” Pelosi said in a letter to her caucus on Sunday. “We can then send it to the president and move quickly to distribute its life-saving resources.” Read more from Erik Wasson and Jarrell Dillard.
The plan includes a wave of new spending, an extension of jobless benefits, another round of direct household payments, money for state and local governments and an expansion of vaccinations and virus-testing programs including a national vaccine distribution program for all residents regardless of immigration status.
- It includes $160 billion for vaccine and testing programs to help stop the virus’s spread and ultimately end the pandemic. The plan includes money to create a national vaccine distribution program that would offer free shots to all U.S. residents regardless of immigration status.
- It also includes funding to create community vaccination centers and deploy mobile units in hard-to-reach areas. Money is also directed toward testing efforts, including purchasing rapid-result tests, expanding lab capacity and helping local jurisdictions implement testing regimens
- .A late addition to the legislation would cover 100% of the costs of continuing health insurance through September for laid-off workers.
- The plan would provide paid-leave benefits of as much as $1,400 per week and tax credits for employers with fewer than 500 employees to reimburse them for the cost of the sick time. The bill does not mandate that employers offer paid leave.
Medicaid Expansion Incentives: The relief bill makes a strong bid to bring Medicaid expansion to the 12 states that haven’t yet allowed working-age adults to become eligible for the program. The bill would cover more than the full cost of expansion for the first two years for any nonexpansion states that come on board, according to Edwin Park, a professor at the McCourt School of Public Policy at Georgetown University. The federal share after the first two years would be 90%, as with other expansion states, Park said.
Full expansion in the holdout states could bring health-care coverage to an additional 4 million people, helping reverse a recent upward trend in the number of uninsured in the U.S. The number of uninsured Americans has grown by 2 million since 2016, according to the Department of Health and Human Services. Read more from Christopher Brown.
Medicare Sequestration Concerns: The American Medical Association, which represents doctors, said the relief measure doesn’t help with “two imminent threats to the financial viability of physician practices.” The first being next month’s expiration of a moratorium on the 2% Medicare sequester, and the second being projections that the aid bill will trigger an additional 4% Medicare sequester. Read their letter to congressional leaders here.
Also Happening on the Hill
Two Health-Related Bills Set for Passage: The House, which meets at noon today, will vote on 13 measures under suspension of the rules, which bars amendments, limits debate, and requires a two-thirds majority for passage. Two of the measures touch on health-care issues:
- H.R. 485, which would reauthorize federal child abuse prevention and treatment programs through fiscal 2027. House Education and Labor Chair Bobby Scott (D-Va.) introduced the bill on Jan. 25 and it was referred to his panel. For more, see the BGOV Bill Summary by Danielle Parnass.
- H.R. 1276, which would open up Covid-19 vaccinations at Veterans Affairs facilities to veterans who aren’t enrolled in the VA department’s health-care system. The bill was introduced on Feb. 24 by House Veterans’ Affairs Chair Mark Takano (D-Calif.) and referred to his panel. For more, see the BGOV Bill Summary by Brittney Washington.
Health-Care Hearings This Week:
- The Senate Health, Education, Labor and Pensions Committee plans a hearing on Covid-19 response tomorrow.
- The House Appropriations Agriculture-FDA Subcommittee meets for a hearing tomorrow on the FDA’s Foreign Drug inspections Program.
- The House Judiciary Subcommittee on Crime, Terrorism, and Homeland Security meets for a hearing on Thursday on federal policies and enforcement dealing with controlled substances.
- The House Appropriations Labor-HHS-Education Subcommittee scheduled a hearing on Thursday on Covid-19, mental health, and substance use.
- The House Education and Labor Subcommittee on Workforce Protections meets for a hearing on Thursday on strategies for protecting workers from Covid-19.
More on the Pandemic
Biden to Hold Event With J&J, Merck CEOs: Biden plans to recognize the partnership between Johnson & Johnson and Merck at an event this Wednesday after the competing drugmakers signed a pact brokered by the White House to accelerate U.S. production. He will attend the event with the chief executives of both firms, Press Secretary Jen Psaki said on Friday. It will take place at Emergent BioSolutions, a J&J supplier, in Baltimore, Josh Wingrove and Jennifer Jacobs report.
AstraZeneca Stockpile May Hasten U.S. Push: AstraZeneca has begun stockpiling its Covid-19 vaccine for use in the U.S., offering a potential supply boost that could speed up Biden’s vaccinations timetable if the company gets federal regulators’ emergency authorization.
A company executive said last week that it’s begun production and expects to have 30 million doses ready for U.S. distribution, once it’s greenlit by the FDA. The drugmaker, which developed the vaccine with the University of Oxford, hasn’t yet applied for FDA approval and hasn’t said when it will, but the U.K.-based company expects to produce 15 to 25 million doses each month for the U.S. after that. If the FDA provides its authorization, “we are expecting to start with 30 million,” Ruud Dobber, AstraZeneca’s executive vice president and president of its biopharmaceuticals unit, said last week to CNBC. “We’re already producing at high speed as we speak, so we feel comfortable.” Read more from Josh Wingrove.
N.C.’s Focus on Data Helps Shrink Racial Vaccine Gap: North Carolina is among the best-performing U.S. states when it comes to distributing vaccines evenly among Black and White residents. That’s partly because the state is by far the best at collecting demographic data. About 11% of North Carolina’s Black population has taken at least one shot, compared with 17% of the state’s White population, according to the Bloomberg Vaccine Tracker. That puts North Carolina in fourth place for the smallest spread between the two groups among states with the most comprehensive data sets.
North Carolina’s success is no accident. The state made equity a priority early on, says Mandy Cohen, Secretary of the North Carolina Department of Health and Human Services. To receive shipments, every provider must use the state’s vaccine management system, which mandates demographic data to finish registering someone for a shot. That has helped the state track its progress and target certain populations more effectively, she says. Angelica LaVito has more.
Republican Reopenings Sharpen Political Divide: Decisions by two Republican governors to remove all coronavirus restrictions in their states have reignited the political debate on the pandemic response, elevating it as a campaign issue this year and in 2022. Republicans Greg Abbott of Texas and Tate Reeves of Mississippi announced last week they’re eliminating state mask mandates and allowing businesses to reopen at full capacity, setting expectations for other GOP-led states to follow suit. The moves drew dire warnings from Democrats and health officials that they risk igniting another spike in cases and deaths and stood in stark contrast to Biden’s cautious approach to getting the U.S. back to normalcy. Read more from Mark Niquette and Jonathan Levin.
U.S. Warming Up to Vaccines, Survey Finds: Covid-19 vaccine hesitancy in the U.S. is ebbing, according to Pew Research. While 39% said in November they probably or definitely would not get a shot, that number declined to 30% in a Feb. 16-21 Pew poll published Friday. Among U.S. adults, 69% are receptive to a vaccine, including 19% who’ve already gotten at least one dose. That compares with 60% in November, when no vaccine was authorized yet in the U.S. Around three-quarters agreed widespread vaccination would help the economy. Read the report here.
- Related: ‘Hassle Factor’ And Distrust Shadow Wide U.S. Vaccine Hesitancy
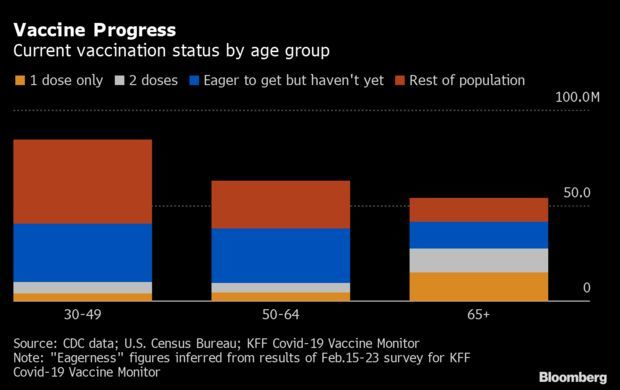
- More than half of Americans 65 years old and over have gotten a Covid-19 vaccination so far, but lingering hesitancy means inoculating the country’s most vulnerable age group is about to get much harder. At least 27.5 million of the 54.1 million Americans in the 65-plus group have received at least one dose, according to the latest update from the U.S. Centers for Disease Control and Prevention. Read more from Jonathan Levin.
More U.S. Headlines:
- Past Big Cities, Independent Pharmacies ‘Begging’ for Doses
- N.Y. Lawmakers Clear Bill to Strip Cuomo’s Pandemic Powers
- Gov. Murphy Missed Early Chances to Halt Covid, Doctor Says
- FDA Warns Against Using Parasite Drug as a Covid Treatment
- FDA Authorizes Adaptive Biotechnologies’ T-Detect Virus Test
- FDA Authorizes Cue Health’s Over-The-Counter Covid-19 Test
- USDA Watchdog Probes Safety Measures for Meat Inspectors
More Global Headlines:
- EU Nations Got a Third of Moderna Shots So Far, Data Show
- Worst Covid-19 Crisis Unfolding in Brazil, Where No Fix Works
- London Is Lagging on Vaccines and the U.K. Can’t Afford That
- Moderna in Deal to Ship 13 Million Vaccines to the Philippines
- Mauritius Halts Inbound Flights After Six Local Covid-19 Cases
What Else to Know Today
Organ Transplant Groups Push to Alter Trump Rule: The transplant medical community is calling on the Biden administration to rework a Trump-era organ procurement rule that they say could be detrimental to the donation process. The rule is designed to make more human organs available for transplant and establishes stricter quality requirements for procurement groups to get federal reimbursements. But it also “injects forced competition” by forcing the groups to “compete for their service area,” a letter to the Centers for Medicare and Medicaid Services says. Read more from Tony Pugh.
Amarin Gets Outside Help in High Court Drug Patent Bid: The Federal Circuit’s approach to evaluating patents will undermine the incentives lawmakers created for developing innovative medications unless the U.S. Supreme Court steps in, a health policy organization told the high court in an amicus brief. Amarin Pharma wants the top court to revive its six canceled patents on the heart treatment Vascepa, which were not set to expire until 2030, Perry Cooper reports.
More Headlines:
- Teva Drug-Label Case Spurs Fresh Litigation as Judges Weigh Redo
- Hospital Debt-Collection Lawsuits Stir New Ways to Push Back
- Caring After Mental Health a Must: IRS Chief Counsel Attorney
- Biotech Firms Lost Roughly $85 Billion in Worst Week in a Year
- Express Scripts Fails to Defeat Louisiana Prescription Fee Case
- Teva Has to Face Patient Claims Over Large Vial Propofol Sales
- Gilead’s Yescarta Receives FDA Approval, With Boxed Warning
- Seqirus’ Flu Vaccine Gets FDA’s OK for Broader Age Indication
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.