HEALTH CARE BRIEFING: Biden Touts Businesses’ Vaccine Tax Credit
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President Joe Biden called on employers to use a tax credit to provide paid time off to workers to get vaccinated and for businesses to do more to boost the inoculation effort as the U.S. seeks to get shots in more arms.
Biden touted the U.S. achieving its goal of giving 200 million vaccine shots in his first 100 days in office, while also pivoting to a new phase of the campaign by calling on businesses to make vaccination as accessible as possible. He requested all employers in the U.S. to offer full pay to workers to get a shot, and touted an incentive included in his first Covid-19 relief package that provides tax credits for workers’ wages in companies with fewer than 500 employees.
The tax credit—covering 100% of up to $511 in daily wages per employee for those small- and medium-sized firms—is intended to push people to get vaccinated, which in turn encourages others to get a shot, said administration officials, speaking on condition of anonymity.
“I’m calling on every employer—large and small, in every state—to give employees the time off they need to get vaccinated,” Biden said. “The time is now to open up a new phase of this historic vaccination effort,” he said, noting that many had not been eligible to get vaccinated until recently. “If you’ve been waiting for your turn, wait no longer.” Read more from Josh Wingrove and Nancy Cook.
- Also yesterday, Biden said the U.S. wants to share Covid-19 vaccines with other countries but won’t send doses abroad until it has sufficient supply domestically. “We are looking at what is going to be done with some of the vaccines that we are not using. We’ve got to make sure they are safe to be sent,” he said. “And we hope to be able to be of some help and value to countries around the world,” Josh Wingrove and Jordan Fabian report.
- Meanwhile, the Biden administration is weighing an appeal from progressive Democrats to accelerate global access to Covid-19 vaccines by supporting a waiver of intellectual-property protections, a move opposed by big drugmakers. Read more from Eric Martin and Susan Decker.
HHS Pulls Trump-Era Vaccine Injury Payment Rule: The HHS yesterday pulled a Trump-era rule that would have made it harder for Americans who suffer shoulder injuries or faint after vaccination to get compensation. The Health and Human Services Department under Trump issued the rule one day before Biden was sworn in, and it would have taken effect on Feb. 22. The move reflects the Biden administration’s efforts to provide more incentives for people to get a Covid-19 vaccine as the supply expands. Read more from Fawn Johnson and Jacquie Lee.
Happening on the Hill
Hearings on the Hill:
- Biomedical Research: The Senate Health, Education, Labor and Pensions Committee convenes for a hearing on U.S. biomedical research and foreign influence.
- Policing, Behavioral Health: The Senate Judiciary Subcommittee on Criminal Justice and Counterterrorism scheduled a hearing on behavioral health and policing.
Lawmakers Agree to Prolong Fentanyl Ban: Congressional leaders are pushing to extend a ban on highly addictive fentanyl analogues, with the promise that lawmakers will then turn to finding a permanent solution to the overdose epidemic. A House-passed six-month extension of the ban, which expires on May 6, left members of both parties unhappy. Republicans want to make permanent the government’s power to classify fentanyl-like drugs among the most-controlled substances. Democrats say such policy is an extension of the war on drugs, which disproportionately hits communities of color.
The House passed the extension (H.R. 2630) by voice vote yesterday. The Senate is set to debate a similar bill (S. 1216) that would extend the ban until July 2022, but no votes have been scheduled. Lawmakers said they’re reluctantly supporting the extension and wary over whether a solution to rising overdose deaths can be found by the end of October. Read more from Alex Ruoff.
Drug Pricing Legislation Unveiled: Amid preparations for Biden’s next infrastrastructure package, House leaders plan to reintroduce their signature drug pricing bill today, which would direct the government to demand lower prices from drugmakers. The bill, slated as H.R. 3, would direct the government to negotiate prices of drugs and biological products that lack competition from generic drugs or biosimilars, as well as insulin. It would also expand Medicare’s benefits and create a $2,000 out-of-pocket cap for Medicare prescription drug plan beneficiaries, Alex Ruoff reports.
Democrats Aim to Lower Child Care Costs: Sen. Patty Murray (D-Wash.) and Rep. Bobby Scott (D-Va.) will introduce legislation today to make child care free for the lowest-income families and cap costs for middle-income families. The Child Care For Working Families Act is expected to be a blueprint for the American Families Plan, the proposal expected from the White House for massive new social spending. Read more from Andrew Kreighbaum.
CMS Pick Slowed Over Medicaid Fight: Sen. John Cornyn (R-Texas) is delaying fast confirmation of Biden’s choice to oversee Medicare and Medicaid, according to the senator’s spokesperson. Cornyn placed a hold on the nomination of Chiquita Brooks-LaSure to be head of the Centers for Medicare and Medicaid Services in response to the Biden administration’s decision to revoke a 10-year extension of Texas’s Medicaid waiver, approved at the end of the Trump administration. Read more from Alex Ruoff.
Panel to Hold Hearing on ‘Long Covid’: Energy and Commerce Chair Frank Pallone Jr. (D-N.J.) and Health Subcommittee Chair Anna Eshoo (D-Calif.) scheduled an April 28 hearing on what’s been called “Long Covid,” when a person’s symptoms persist for weeks and months after the initial infection and potentially after testing negative. The hearing “will be highly instructive in learning more about ‘long COVID’ and the work the NIH, CDC, and the health care and patient community are doing to better understand its long-term effects,“ Pallone and Eshoo said in a statement. Read the statement here.
Telehealth Hearing Announced: House Ways and Means Health Subcommittee Chair Lloyd Doggett (D-Texas) announced a hearing entitled “Charting the Path Forward for Telehealth” for April 28, according to a release.
The Coronavirus Pandemic
J&J Vaccine Plant to Stay on Hold After Inspection: Production at an Emergent BioSolutions facility in Baltimore that’s expected to help make Johnson & Johnson’s coronavirus vaccine will remain on hold, U.S. regulators said, after an inspection turned up several problems. The FDA said in a report posted on its website yesterday that Emergent failed to thoroughly investigate unexplained discrepancies, including the cross-contamination of a Covid-19 vaccine substance batch with ingredients from another shot. The FDA said no vaccine manufactured at the plant has been distributed for use in the U.S.
In response to the findings, J&J said in a statement that it planned to set up a global supply network to produce its shots, and that it would ensure that the concerns raised by the FDA about the Emergent plant are addressed. Emergent spokesman Matt Hartwig said the FDA report will give the manufacturer direction on steps necessary to improve operations. Read more from Riley Griffin and Anna Edney.
Vaccine Waste at 0.1% Fails to Ease the Worries of Doctors: States across the U.S. are reporting little waste of Covid-19 vaccine, in what appears to be evidence that careful handling of doses amid soaring demand is helping get almost every available shot into the arms of Americans. Any global mass-vaccination campaign — such as those for Ebola or measles — reports wastage, or doses ready for injection that aren’t administered. The numbers varied widely last month, such as 6.5% of Covid vaccines wasted in India to 1.8% in Scotland. Wales, in a weekly report on April 11, listed no more than 0.9% of doses as “not suitable for use” among the Pfizer, Moderna and AstraZeneca formulas. As of April 12, only 0.12% wastage has been reported to the U.S. Centers for Disease Control and Prevention, which collects reports on how many doses are spoiled, expired or otherwise unusable, said CDC spokeswoman Kate Fowlie. Read more from Elise Young.
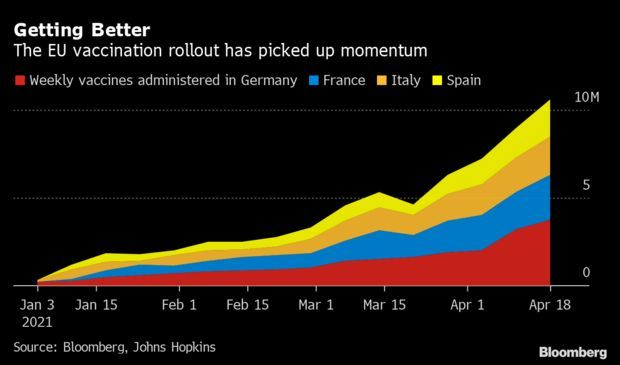
Europe Is Turning a Corner on Vaccinations: The European Union’s long-awaited Covid-19 shot surge is finally here, raising hopes the bloc can bring the coronavirus under control and reopen economies faster than expected. The inflection point came this month, with Germany almost doubling the pace of vaccinations after an increase in supplies and the decision to let general practitioners administer shots in their regular offices. And France, Italy and Spain are following a similar trajectory. Read more from Tim Loh.
More Headlines:
- Herd Immunity Is Humanity’s Great Hope, and It’s Proving Elusive
- U.K. in Regular Talks With U.S. Over a Potential Travel Corridor
- Virus Workplace Rule Still MIA Three Months After Biden Order
- Japan Sees Tokyo, Osaka Virus Emergency Decision This Week
- Modi Urges India’s States to Shun Lockdowns as Virus Surges
- Thailand Sees ‘Stroke-Like’ Symptoms Tied to Sinovac’s Shots
- Where Covid Kills the Young: Brazil Shows What May Await Others
What Else to Know Today
Employers Demand Eased Telehealth Rules: The Biden administration should take steps to boost access to telemedicine, reduce costs for out-of-network Covid-19 testing, and increase vaccine availability to help bring employees back to work, a group of large employers urged. The ERISA Industry Committee called on the Department of Labor, the Department of Health and Human Services, the Treasury Department, and the IRS to provide immediate pandemic relief to employees, such as by permanently easing telehealth restrictions and protecting the workforce from high medical costs. Read more from Sara Hansard.
U.N. Urged to Pressure U.S. on Menthol: Almost 100 advocacy groups are asking the United Nations to look at the adverse effects of menthol cigarettes on African Americans’ health as a human-rights issue, a move that ramps up pressure on the U.S. government to ban the minty flavored tobacco products. The request is addressed to a United Nations committee that aims to end racial discrimination. The effort’s leaders include the African American Tobacco Control Leadership Council and Action on Smoking and Health. Read more from Tiffany Kary.
- Illinois Democratic lawmakers, including House Oversight and Reform Economic and Consumer Policy Chair Raja Krishnamoorthi, Rep. Bobby Rush, and Senate Majority Whip Dick Durbin, separately called for the FDA to ban menthol-flavored cigarettes to “protect both public health and racial equity” in a letter yesterday, citing data that 70% of African American youth age 12 to 17 use menthol cigarettes.
More Headlines:
- Biden Aide’s Brother Lobbied Admin. for Health Care Firms: CNBC
- Medicare Agent Marketplace Sought to Aid Beneficiaries
- Five Fresh Legal Faces to Know in Healthcare and Life Sciences
- Oklahoma Lawmakers Vote to Limit Abortion Procedures to OB-GYNs
- FDA Pulls Appeal to Required Redo of ‘Frankenfish’ Salmon Review
- California Top Court Passes on J&J, Teva Opioid Subpoena Fight
With assistance from Alex Ruoff
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.