HEALTH CARE BRIEFING: Biden Duo’s Nudge Eased Merck-J&J Logjam
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
It was Valentine’s Day, and top Biden administration officials were concerned that Johnson & Johnson’s coronavirus vaccine was behind schedule.
In a phone call that Sunday between J&J Chairman and Chief Executive Officer Alex Gorsky and Biden’s Covid-19 response coordinator, Jeff Zients, and science adviser David Kessler, Kessler implored Gorsky to ramp up production of the company’s shot.
He drew a comparison with Robert Wood Johnson, son of one of the company’s founders, who became known as “General” Johnson after leading a World War II effort to involve small businesses in the production of wartime supplies.
This, too, is a wartime effort, Kessler told Gorsky, and the status quo isn’t enough. Scheduled to last 15 minutes, the call extended to 90, according to people familiar with the matter. Afterward, J&J’s executive vice president, Kathy Wengel, called the Biden team back and said the company was on board, the people said.
The push from the president’s advisers reinvigorated simmering talks that led to a tie-up between the company and its competitor Merck & Co., which also struck a separate deal with the U.S. government to help it speed up J&J’s production.
President Joe Biden on Wednesday will celebrate the Merck and J&J partnership, which he has said his administration brokered to accelerate manufacturing of J&J’s shot, the third vaccine the U.S. has authorized to prevent Covid-19. Biden has described the deal as one of several accomplishments that will lead to the U.S. having enough vaccines for every American adult by the end of May, two months earlier than he’d previously predicted. Read more from Josh Wingrove and Riley Griffin.
Happening on the Hill
Biden Stimulus Nears Final Approval With House Vote Today: The House is poised to send the $1.9 trillion Covid-19 relief plan to President Joe Biden for his signature, providing an economic boost that will last long after $1,400 stimulus checks start arriving in Americans’ accounts this month. With four days until supplemental unemployment benefits begin running out, House Democratic leaders expect passage Wednesday morning.
The bill is far bigger than initial Wall Street expectations of what could be accomplished in a closely divided Congress. It provides a template for a potential longer-term expansion of an American social-safety net that has long been much smaller than its European counterparts. Democrats say the near-$110 billion temporary expansion of the child-tax credit will help cut child poverty in half, while tax forgiveness on jobless benefits and student-debt relief will give help to millions more. Read more from Erik Wasson and Katia Dmitrieva.
House Floats Proxy Voting Post-Crisis, Hoyer Says: House Majority Leader Steny Hoyer (D-Md.) said there will be discussions about extending proxy voting after the Covid-19 pandemic ends, suggesting it may be used for members who are sick, dealing with the death of a relative, or on maternity leave. “There is no magic about being in a particular room when you vote,” Hoyer said yesterday, Emily Wilkins reports.
Medical Research Advocates Brace for Blunt’s Exit: Medical research advocates will likely have to push harder after 2022 for the NIH funding increases they’ve enjoyed over the past several years after the departure of Sen. Roy Blunt (R-Mo.). Blunt announced that he won’t seek re-election when his term expires in 2022. The fourth-highest ranking Senate Republican serves as the top Republican on the panel that oversees medical research funds, where he made National Institutes of Health research one of his main priorities. Read more from Jeannie Baumann.
The Coronavirus Pandemic
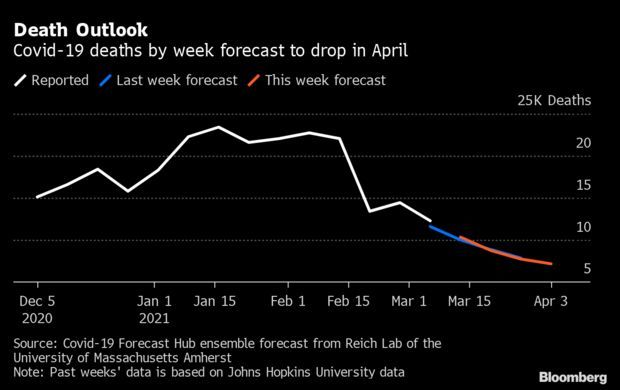
Death Forecast Drops as Pandemic Indicators Improve: The outlook for Covid-19 deaths in the U.S. is expected to fall in the coming weeks to levels not seen since November. The U.S. is forecast to record 7,166 weekly deaths for the week ending April 3, according to new forecasts from the University of Massachusetts’ Reich Lab Covid-19 Forecast Hub. That’s fewer than one third of the peak reported in January. Unlike deaths, the weekly cases could rise in the coming weeks, however. Aggregate forecasts for the week ending March 27 ticked up to 319,703 from 305,450, according to the hub. Read more from Nic Querolo.
CDC’s Cautious Approach to Travel Criticized: Lack of comprehensive evidence on Covid-19 transmission is prompting the CDC to be overly cautious on its guidance to vaccinated people who want to travel, epidemiologists and doctors argued. The Centers for Disease Control and Prevention didn’t include any changes to travel guidance for fully vaccinated individuals in its guidelines released yesterday. “I don’t see any point why vaccinated people can’t travel,” said Monica Gandhi, a University of California medical professor. Shira Stein has more.
Prepare for Vaccine Ramp-Up in May, Messonnier Says: Communities should prepare now to boost vaccination efforts in the coming months when there will be enough doses available for every American adult who wants one, said Nancy Messonnier, head of the National Center for Immunization and Respiratory Diseases in the Centers for Disease Control and Prevention, at a health policy conference yesterday. Read more from Jeannie Baumann.
- BioNTech could have capacity to make 3 billion doses of Covid-19 vaccine with U.S. partner Pfizer next year, the German company’s chief executive officer said, making their pioneering shot far more widely available around the world. “In principle, we could further increase manufacturing capacity,” BioNTech CEO Ugur Sahin said yesterday in an interview with Bloomberg TV. “It depends on demand, it depends on factors such as if an additional boost to vaccinations is required.” Read more from Naomi Kresge and Matthew Miller.
More Headlines:
- New York Expands Vaccine Eligibility to Everyone 60 and Older
- Algorithms Link Up Vaccine Seekers With To-Be-Wasted Doses
- EU’s Vaccine Pass Might Open Door to Russian, Chinese Shots
- How Africa Can Save the World From a Neverending Pandemic
- Vaccine Gains Bring a Despised President Back From the Brink
What Else to Know Today
HHS Eyes More Time on Trump Insulin Rule: The HHS yesterday proposed further delaying a Trump administration drug rule that requires community health centers to pass on all their insulin and epinephrine discount savings to patients. Centers that don’t pass on the savings wouldn’t qualify for federal grants. Biden’s Health and Human Services Department delayed the effective date of the rule until March 22. It’s now proposing July 20. Jacquie Lee has more.
U.S. Adds More Time to Value-Based Care Privacy Rule: A proposed rule that would modify a health privacy law to make it easier for doctors and hospitals to coordinate patient care was pushed to May 6 from March 22, the HHS said yesterday. The proposed rule would modify the law’s standards that may impede the transition to value-based health care by restricting care coordination and case management communications among hospitals, physicians, and other health care providers, as well as insurers. Read more from Fawn Johnson.
More Headlines:
- ‘Chaos’ Would Come From HHS Review of Every Rule, Lawsuit Says
- Marinus Pharma Gains on Phase 3 Trial of Seizure Drug for Tumors
- FDA Surprise Pushback May Cause Concern for Biotech, Truist Says
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.