HEALTH CARE BRIEFING: Biden Delays June Target to Ship Vaccines
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President Joe Biden is delaying a target date for overseas shipments of 80 million doses of coronavirus vaccines donated by the U.S., with logistical hurdles and millions of shots produced by AstraZeneca still in a safety review.
Biden’s White House yesterday announced a list of countries that would receive 55 million vaccine doses, after previously detailing the first tranche of 25 million. In total, three-quarters of the 80 million will be sent through COVAX, the World Health Organization-backed vaccine procurement effort.
The announcement, however, dials back the initial U.S. commitment. Biden once said he’d “send” 80 million doses by the end of June, and is now vowing only to “allocate” them by then, suggesting shipments will stretch into July or beyond. “The Biden-Harris administration is committed to working with U.S. vaccine manufacturers to produce more vaccine doses to share with the world,” White House Press Secretary Jen Psaki said.
She said the shipments have been delayed by a host of logistical issues overseas, including making sure countries receiving the vaccines have sufficient supplies to administer them—like needles and alcohol pads—and can clear them through customs fast and keep the shots in cold storage.
“What we’ve found to be the biggest challenge is not actually the supply,” Psaki told reporters at a briefing. “This is a Herculean logistical challenge.” Read more from Josh Wingrove.
More on the Pandemic
Covid-19 Rebounds in U.S. South: Covid-19 transmission is accelerating in several poorly vaccinated states, primarily in the South, and more young people are turning up at hospitals. The data present the clearest sign of a rebound in the U.S. in months. In Utah, Missouri, and Arkansas, the seven-day average of hospital admissions confirmed Covid-19 has increased by more than 30% in the past two weeks, according to the Department of Health and Human Services. Read more from Jonathan Levin.
Variant Seen in Counties With Low Vaccine Uptake: Early evidence suggests that the highly contagious Delta variant, which has prompted concern worldwide as it leads to new surges of Covid-19 across the globe, is spreading around undervaccinated pockets of the U.S. The genomics firm Helix found that cases of the variant first documented in India appear to be growing much faster in counties with lower vaccination rates than in areas that have higher rates. Read more from Kristen V. Brown.
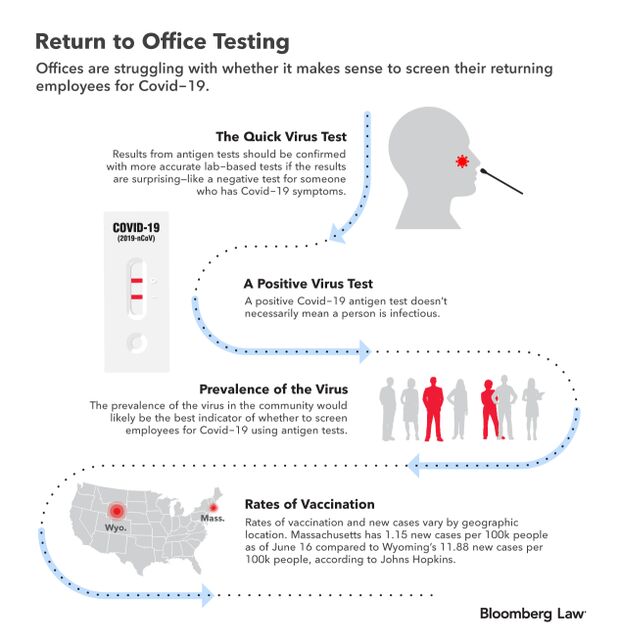
- Businesses are struggling with the best way to transition back to in-person offices, and the science behind fast coronavirus tests makes deciding whether to screen returning workers tricky. Many employers aren’t requiring their employees to get vaccinated at all. Experts instead suggest employers focus on how prevalent the virus is in their communities to decide whether relying on rapid antigen tests makes sense for them. Read more from Jacquie Lee.
Nursing Home Death Suits: The Third Circuit tomorrow will become the first federal appeals court to hear oral arguments on whether state-law Covid 19-related wrongful death suits against nursing homes belong in state or federal court. Similar appeals are pending in the Second, Fifth, Ninth, Eleventh, and D.C. circuits from federal district court decisions rejecting nursing homes’ assertions that the federal officer removal law gave them authority to hear the cases. Mary Anne Pazanowski has more.
Smithfield Sued for Profiting From Covid-Led Meat Shortage Fears: Consumer advocacy group, Food & Water Watch, is suing Smithfield Foods, the top U.S. pork producer, for allegedly fueling fears of a meat shortage during the pandemic to boost demand and prices for its products even as it had ample reserves. The lawsuit also alleges the company misled the public about safety measures for its workers. Read more from Mike Dorning.
More Headlines:
- Health-Care Firms Must Follow OSHA Covid-19 Rule by July 6
- U.S. Lowers Ireland Travel Warning Over Virus by One Notch
- Glaxo, Vir Data Affirm Sotrovimab Cuts Covid-19 Death Risks
- Vaccine ‘Honor System’ in U.S. Leaves False Sense of Security for Businesses
- Reopened Offices Fear Faulty Screening Tests as Virus Recedes
More Global Headlines:
- Johnson Says U.K. Virus Data Looking Good for Lifting Curbs
- Canada Eases Quarantine Rules for Citizens Crossing Border
- South Africa, France Plan First African mRNA Vaccine Factory
- S. African Regulator Says Sinovac Application in Later Stages
- Indonesia’s Covid-19 Count Hits 2 Million, as Hospitals Max Out
Happening on the Hill
Vaccines: The Senate Health, Education, Labor and Pensions Committee scheduled a hearing on vaccines and the effort to end the Covid-19 pandemic.
Health Bills on Tap: The House is scheduled today to consider two health-care measures under suspension of the rules—which bars amendments, limits debate, and requires a two-thirds majority for passage:
- Newborn Health Screenings: Federal programs helping states expand their newborn screening programs would be reauthorized for five years under H.R. 482. The legislation would also fund quality assurance monitoring of the laboratories involved in newborn health screenings. The House Energy and Commerce Committee hasn’t considered the bill. For more, see the BGOV Bill Summary by Christina Banoub.
- Tribal Health Data: Federal agencies would have to share public health data with American Indian tribes under H.R. 3841, which also would reauthorize the National Center for Health Statistics for five years. The House Energy and Commerce Committee hasn’t acted on the bill. For more, see the BGOV Bill Summary by Christina Banoub.
Panel to Weigh Assistant HHS Chief Pick: Senate Finance Chairman Ron Wyden (D-Ore.) yesterday announced he will convene a hearing on June 24, to consider the nomination of Melanie Egorin to be assistant HHS Secretary.
Bipartisan Duo Push for Diabetes Tech Access: Sens. Susan Collins (R-Maine) and Jeanne Shaheen (D-N.H.), the co-chairs of the Senate Diabetes Caucus, unveiled legislation that would “expand seniors’ access to groundbreaking diabetes technologies, such as the artificial pancreas and implantable continuous glucose monitoring systems,” according to a statement. The bill would establish a task force to address “barriers that seniors face” in accessing such tech.
What Else to Know Today
HHS Fills Drug Discount Dispute Board: The Biden administration appointed panelists for a long-awaited forum to hear disputes within a government program for providing steep drug discounts to health centers treating lower-income patients. HHS Secretary Xavier Becerra has ordered the appointment of members to the 340B Administrative Dispute Resolution Board, a group of health centers said in a filing in a lawsuit over the discount forum. Read more from Ian Lopez.
Teva to Settle Mississippi Drug-Price Case: Teva Pharmaceutical said it had agreed to pay the state of Mississippi $925,000 to resolve claims that the company fixed prices of generic drugs, and that it is discussing potential pacts with other states. In 2019, Mississippi was part of a large group of U.S. states that sued Israel-based Teva and other generic-drug makers, alleging a conspiracy to divide up customers and increase the cost of certain medications. Teva said under the terms of the agreement with Mississippi Attorney General Lynn Fitch it didn’t admit guilt, wrongdoing or liability. Read more from Riley Griffin.
Medicare Group Names Mary Beth Donahue as CEO: Better Medicare Alliance, a research and advocacy group that supports Medicare Advantage, announced health policy veteran and patient advocate Mary Beth Donahue will become its president and CEO this summer, according to a statement. Donahue served as chief of staff to former HHS Secretary Donna Shalala during the “critical early days of Medicare Advantage.” Read the statement here.
More Headlines:
- Justices Pass on Second Obamacare Cost-Sharing Payment Fight
- Supreme Court Turns Away AbbVie Androgel ‘Sham’ Patent Spat
- Supreme Court Rejects Amarin Bid to Revive Patents for Vascepa
- Kentucky County Opposes Abortion Clinic Buffer Zone Challenge
- Bayer Sends U.S., EU Application for Oncology Treatment Combo
- Caps on Medicare-Funded Graduate Medical Education at Teaching Hospitals (GAO)
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.