HEALTH CARE BRIEFING: Becerra Makes Nomination Battle Personal
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
California Attorney General Xavier Becerra will defend his experience to senators on the Finance Committee at a confirmation hearing today, a day after facing questions from the Senate health panel on his qualifications to be secretary of Health and Human Services under President Joe Biden.
Becerra is betting that a personal touch will secure enough votes to assure his nomination in coming weeks, a day after he sought to retain the support of abortion-rights Democrats while also wooing the GOP during his meeting with the Senate panel that oversees health.
Outside his nomination hearings, Becerra has met with key Republican senators, even as several staunch conservatives in Congress and anti-abortion groups publicly condemned his nomination. And he’s made a point of talking with lawmakers about their home-state issues, sayinghe could help them as head of the Department of Health and Human Services.
Becerra’s resume is highlighted largely by fights—legal battles he waged as California’s attorney general against Trump administration policies. But to clear the Senate, he has focused on personal issues, including his own.
“Everything I do, including this, is a family affair,” Becerra said yesterday at his first confirmation hearing, mentioning that his mother was a Mexican immigrant and his father died early this year.
Becerra has spoken privately with Republican Sens. Mike Braun (Ind.), Bill Cassidy (La.), John Cornyn (Texas), Mike Crapo (Idaho), Lisa Murkowski (Alaska), and Rob Portman (Ohio) since being nominated, according to the lawmakers’ public comments and spokespeople from their offices. All sit on one of the two committees tasked with advancing his nomination. Read more from Alex Ruoff.
Becerra’s Day 1 Recap: Yesterday Becerra testified to the Senate Health, Education, Labor, and Pensions Committee, where Sen. Richard Burr (R-N.C.) stopped short of saying that he’ll oppose the Health and Human Services pick but questioned his qualifications. “I do come with an open mind, and now the job is up to you,” he said. Read more about the hearing from Shira Stein, Alex Ruoff, and Emma Court.
White House Coronavirus Briefing: The White House Covid-19 Response Team will hold an 11 a.m. briefing today.
Also Happening on the Hill
Stimulus Update: The House will vote Friday on Biden’s American Rescue Plan, Majority Leader Steny Hoyer (D-Md.) said last night. The House Rules Committee is set to consider the chamber’s $1.9 trillion coronavirus relief bill Friday. Read the bill text and hearing information here. Sen. Susan Collins (R-Maine) said yesterday she doesn’t see a single GOP vote for the legislation. She suggested that though President Joe Biden himself was interested in bipartisan talks, they were halted by aides. Separately, Biden, at a virtual roundtable with Black essential workers, acknowledged that the vote in Congress will be close, Erik Wasson reports.
Lawmakers Unveil Bill to Create Public Option: Sens. Jeanne Shaheen (D-N.H.), Michael Bennet (D-Colo.) and Tim Kaine (D-Va.) reintroduced legislation yesterday they said would create a public option by expanding on Obamacare and Medicare. The measure would work “within the Medicare framework to establish a Medicare Exchange public option plan in every county in America for individuals and small businesses, providing an additional, affordable option in all communities,” according to a statement.
House Homeland Hearing: The House Homeland Security Committee holds a hearing today to assess the Covid-19 efforts one year into the pandemic.
The Coronavirus Pandemic
Biden Shelves Mass Mask Shipments: Biden will announce a program to send cloth masks to disadvantaged U.S. communities to curb the coronavirus pandemic while deciding for now to shelve a proposal to send masks to every American, according to two administration officials familiar with the plans. The U.S. will probably send millions of masks across the country “very shortly,” Biden said yesterday at a virtual roundtable event with Black essential workers who discussed the pandemic response with him. ”We’re probably going to be sending out an awful lot of masks around the country, very shortly — millions of them,” he said, without elaborating. Shira Stein and Josh Wingrove have more.
Vaccine Supply Strains Set to Ease, Lawmakers Hear: Bottlenecks that have shadowed the country’s immunization campaign could soon begin to ease, vaccine makers said in a Capitol Hill hearing. Moderna said it has received positive feedback from U.S. regulators on a proposal to expand the number of doses of its Covid-19 vaccine in every vial, while Pfizer said it sees output ramping up in the coming weeks. Riley Griffin and Robert Langreth have more.
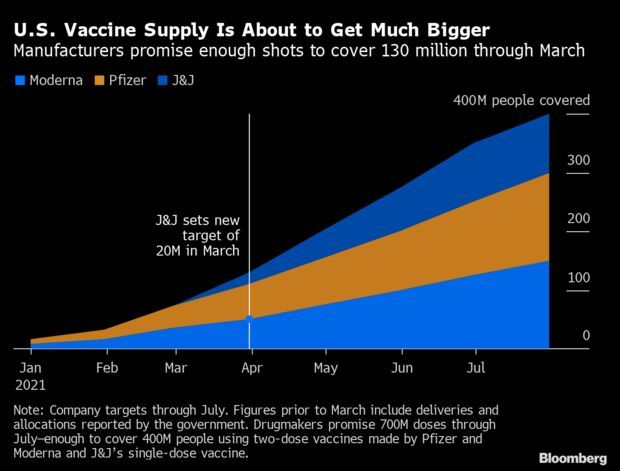
- Also, Johnson & Johnson announced yesterday it’s on track next month to distribute 20 million doses of its one-shot vaccine, adding to a coming surge in vaccine availability, a Bloomberg analysis found. Along with vaccines from Pfizer and Moderna, the delivery targets set through March will be enough to fully vaccinate 130 million Americans. Tom Randall and Drew Armstrong have more.
- The U.S. may issue guidance easing public health protocols for fully vaccinated people, Anthony Fauci, Biden’s chief medical adviser, suggested, amid expectations of a surge in vaccine availability in March. Still, Fauci cautioned that he didn’t want to get ahead of guidance from the Centers for Disease Control and Prevention, which he said could be forthcoming, Bloomberg News reports.
- Related: Regulators to OK Pfizer Request on Vaccine Storage Temps: NYT
NIH to Study Long-Term Covid-19 Effects: The National Institutes of Health is undertaking a new project to study the long-term effects of Covid-19, Director Francis Collins said. “Through this initiative, we aim to learn more about how SARS-CoV-2 may lead to such widespread and lasting symptoms, and to develop ways to treat or prevent these conditions,” Collins said in a statement announcing the study. Read more from Jeannie Baumann.
U.S. Steps Up Genetic Analysis for Variants by 5,500%: The U.S. is analyzing about 14,000 coronavirus cases every week with genetic sequencing to detect quickly spreading variants, Centers for Disease Control and Prevention Director Rochelle Walensky said. That’s up from 250 sequences each week when Walensky took office last month, she told lawmakers on the House Appropriations Labor-HHS-Education Subcommittee panel yesterday morning. Read more from John Tozzi.
- 90-Day Immune Protection Leads to Vaccine Delay Call: Meanwhile, Walensky also said those who’ve had Covid-19 may consider waiting to receive a vaccine to allow others who have no immunity to get the jab. Covid-19 survivors are likely to have “some immune protection up to at least about 90 days,” Walensky said, Kathleen Miller reports.
More Headlines:
- Confusing Data About Efficacy Haunt AstraZeneca’s Covid Vaccine
- Tomorrow’s Hospitals: Smaller, More Flexible, Pandemic-Proof
- Some Health-Care Sector Fortunes Fading With Vaccine Rollout
- Thailand Mulls Waiving Self-Quarantine for Vaccinated Tourists
- Airline Bookings Surge, Buoyed by U.K. Plans to Reopen Travel
- Vaccine Delays Leave Latin America Economies Still in the Mud
What Else to Know Today
Pfizer Suit Tests Crackdown on Copays: A Pfizer challenge to an enforcement push against drug company and charity collaborations is testing the bounds of the government’s scrutiny of illegal kickbacks and showcases the complexity of helping patients pay for high-cost drugs. Justice Department allegations that such arrangements are unlawful have netted the federal government over $1 billion in settlements since 2017. Officials view them as attempts to beat Medicare copay mechanisms’ cost-control functions. Christopher Brown has more.
Generic-Drug Labeling Strategy Likely to Survive Teva Rehearing: A U.S. appeals court panel signaled that it wants to ensure generic-drug makers can continue selling low-cost copycat medicines with limited-use labels as long as they don’t actively promote newer uses discovered by brand-name companies. In an unusual proceeding, a three-judge panel of the U.S. Court of Appeals for the Federal Circuit yesterday heard arguments for the second time over a $235 million verdict won by GlaxoSmithKline Plc over Teva Pharmaceutical Industries’ copy of its Coreg heart drug. Read more from Christopher Yasiejko, Ian Lopez and Susan Decker.
More Headlines:
- Pennsylvania Rule Setting Court for Medical Injury Cases Valid
- ‘Icy Hot’ Pain Patch Marketing Is Deceptive, Rival Firm Argues
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.