HEALTH CARE BRIEFING: Hospitals Call On Congress for $25 Billion
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Hospitals are asking Congress to give them another $25 billion and hand out all previously allotted funds to shore up facilities ravaged by the omicron outbreak. The money would pay for training and extra security as hospitals cope with staff shortages, higher costs and lost revenue, the American Hospital Association said in a letter to congressional leaders.
“We are now in need of additional immediate support from Congress and the administration in order to continue standing strong and to be able to provide timely access to life-saving health care to your constituents,” AHA Executive Vice President Stacey Hughes wrote in a letter dated Jan. 20. “The current surge has impacted hospitals in ways not seen previously.”
The pandemic has slammed hospitals with higher costs both for personnel and supplies, while limiting the day-to-day medical procedures and operations that pay the bills. Some U.S. states and hospitals have paused elective surgeries to make room for coronavirus patients struggling to survive. Meanwhile, earlier rounds of federal funding meant to help tide hospitals over are winding down, and more pressure is looming from changes in Medicare payments.
The hospital group asked Congress to immediately distribute the money remaining in the $178 billion Provider Relief Fund and the $8.5 billion rural hospital fund. No distributions have been made or announced since either the delta and omicron variants caused new spikes in hospitalizations, the group said. AHA also called on Congress to further delay a pending 2% Medicare cut until at least the end of the year, and to pause payments that hospitals owe for receiving advances on Medicare funding for six months. Read more from Lauren Coleman-Lochner.
- BGOV OnPoint—More Covid-19 Aid Eyed: The AHA letter comes as lawmakers weigh additional Covid-19 aid to address omicron, even as Republicans raise questions about money left over from prior pandemic relief laws. Democratic leaders said they’re expecting a request from the Biden administration for more virus aid, which could hitch a ride on fiscal 2022 spending legislation. Read more from Michael Smallberg.
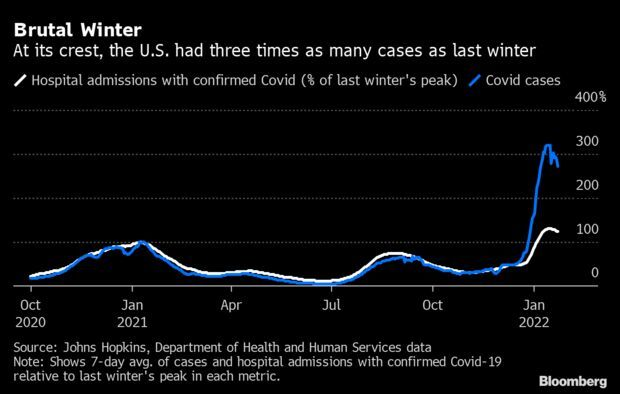
- The U.S. is reporting the most Covid-19 deaths in about 11 months, but cases and hospitalizations signal that the nation is turning a corner. The seven-day average of reported Covid-19 deaths hit 1,975 on Friday, the most since Feb. 25, new data from the Centers for Disease Control and Prevention show. During the winter peak in January 2021, the daily death average topped 3,400. Read more from Jonathan Levin.
More on the Pandemic
FDA Halts Covid-19 Drugs Seen Ineffective Against Omicron: U.S. regulators restricted the use of a pair of Covid-19 monoclonal antibody therapies after scientific evidence suggested they’re unlikely to be effective against the omicron variant. The Food and Drug Administration said in a statement yesterday that it’s decided to limit access to the treatments, which are made by Eli Lilly and Regeneron, amid the recent surge in omicron infections. As a result of the FDA decision, the federal government will pause shipments of the drugs. Read more from Fiona Rutherford.
- Public health agencies need to spur greater use of well-designed drug studies to get quicker answers about potential treatments for the next pandemic, the FDA’s biologics chief said. Peter Marks’ remarks echo calls from acting agency FDA chief Janet Woodcock for a revamped clinical trials network that can quickly randomize patients to find evidence on which treatments work. Jeannie Baumann has more.
Churches’ Challenges to Colorado Covid-19 Policies Dumped: A lawsuit challenging Colorado’s restrictions on religious worship amid the pandemic is over because the state has lifted the limitations and isn’t likely to reimpose them, a federal appeals court said yesterday. The suit alleging that restrictions like social distancing and masking requirements violate the U.S. Constitution’s free exercise clause is moot because the state’s no longer enforcing them against houses of worship, the U.S. Court of Appeals for the Tenth Circuit said in the unpublished opinion. Read more from Mary Anne Pazanowski.
Fourth Shot Gives Protection From Infection, Israel Says: A fourth vaccine dose for older adults leaves them better protected against coronavirus infection than peers who received three shots, a study released by Israel’s health ministry found. The researchers found that those who had the fourth dose had twice the protection from infection as the others, and at least three times the protection from severe illness. Read more from Alisa Odenheimer.
- Separately, Pfizer and BioNTech said results from two laboratory studies demonstrate that three doses of the Pfizer-BioNTech Covid-19 vaccine elicited antibodies that neutralize the omicron variant, Susanne Barton reports.
More Headlines:
- Siemens Tapped to Supply 50 Million Rapid At-Home Tests in U.S.
- Alberta’s Kenney Talks to Governors on Ending Trucker Jabs Rule
What Else to Know Today
Califf on Path to Win FDA Chief Role Despite ‘No’ Votes: Robert Califf’s previous stint as FDA chief will help him secure the role once again despite growing lawmaker opposition over his drug industry ties, former agency officials say. At least five Democratic senators have said they oppose Califf as he awaits a Senate vote, and it’s unlikely that he will see the same level of overwhelming support he got in 2016. But policy analysts say his record of letting science guide decisions will outweigh concerns over how Califf might act on opioids or the abortion drug mifepristone. Celine Castronuovo and Alex Ruoff have more.
Pharma Chips Away at Discounted Drugs While Battling HHS: Major pharmaceutical companies continue cutting off for-profit pharmacies from drug discounts aimed at helping lower-income patients even as they fight the HHS in courts across the country—a trend with no end in sight. AbbVie, Amgen, and Bristol Myers Squibb are among the latest companies to limit price cuts they offer through the government’s 340B program, which requires drug companies to give discounts to providers serving vulnerable populations in exchange for their drugs participating in Medicaid and Medicare Part B. Ian Lopez has more.
More Headlines:
- Change Said to Weigh Asset Sale to Aid UnitedHealth Merger
- Shock Therapy Memory-Impairment Lawsuit Partly Advances
- Mustang Bio Tumbles as FDA Holds on New Drug Application
- ALS Group Taps Veteran Advocate Dalle Pazze as Its New CEO
- Cassava Sued Over Alzheimer’s Drug Data Manipulation Allegations
With assistance from Alex Ruoff
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.