HEALTH CARE BRIEFING: Patent Impasse Blocks Vaccinating Billions
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Covid-19 vaccines look set to protect millions of citizens of the world’s richest countries in the coming months. But inoculating the rest of the planet’s population may mean finding a way around an impasse over intellectual property.
Representatives from all 164 member states of the World Trade Organization met last week in Geneva to discuss a proposal from India and South Africa to waive broad sections of the WTO’s intellectual property rules and to try to forge an agreement on how patents developed in the race against Covid-19 should be recognized.
The meeting ended without consensus, leaving poorer countries who sponsored the proposal frustrated and legal protections for vaccines intact. That may be a victory for patent protection advocates, but pressure for change will only grow if billions of people in poorer countries go unvaccinated while the rich world starts getting a steady flow of doses from Pfizer and BioNTech, Moderna and AstraZeneca.
“With the biggest health crisis we’ve experienced, we’re still not able to find alternative ways of dealing with the IP issues when everyone’s lives are at stake,” said Tahir Amin, executive director of the Initiative for Medicines, Access & Knowledge, an organization promoting better access to drugs. “You’ve got the advocates saying ‘Let’s knock the wall down,’ and then you’ve got the investors who say ‘If we open the door it’s like the floodgates.’ We have to be smarter than that.”
A patent gives a drugmaker exclusive rights to manufacture a vaccine it developed, also providing it the power to charge a price that covers the costs of research and development. Their profit margin per dose, however, depends on the urgency of the situation, and amid a pandemic, charging anything more than development costs is bound to be controversial. India’s proposal would require that the waiver remain in place until there’s been widespread vaccination and the majority of the world’s population has developed immunity.
Whether it’s possible to reconcile will only be clear as the pandemic plays out. The European Union and U.S., home to leading drugmakers, are vehemently opposed to the proposition, though pricing may offer some room for negotiation. Read more from Hugo Miller and Susan Decker.
Moderna’s Vaccine Found Safe, Effective: Moderna’s vaccine is safe and effective in people ages 18 and older, U.S. regulators said yesterday, clearing a path for a second shot to quickly gain emergency authorization and add to the country’s massive immunization campaign. The Food and Drug Administration’s staff said in a report that the experimental candidate is 94.1% effective at preventing symptomatic cases of Covid-19, confirming earlier results published by the company. The report comes ahead of a meeting tomorrow of FDA advisers who will weigh whether to recommend authorization before a final FDA decision.
The agency doesn’t need to follow the advice of the independent vaccine experts, although it often agrees with its advisory panels. Last week, the agency authorized a similar vaccine from Pfizer and BioNTech after an advisory panel voted 17-4 to support its emergency deployment, which got underway nationwide this week. Read more from Anna Edney and Robert Langreth.
Related:
- Moderna to Offer Covid-19 Shots to Volunteers Who Got Placebo
- Pfizer Offers Covid Shots to Health-Care Workers Who Got Placebo
States Get $227 Million for Vaccine Distribution: The federal government will provide states and territories almost $227 million to distribute Covid-19 vaccines and track the virus, the HHS announced yesterday. The Centers for Disease Control and Prevention will award $140 million to all 50 states and additional jurisdictions for vaccine preparedness and $87 million for Covid-19 tracking and testing. Read more from Jacquie Lee.
More on the Vaccine:
- Trump to Tout Vaccine But Hasn’t Decided When He May Get It
- Trudeau Boosts Vaccine Push, Nabs Early Doses From Moderna
- Pfizer-BioNTech Covid-19 Shot to Get European Review Next Week
- Permit Vaccinations at Eye Clinics, Optometrist Group Tells Azar
- Walgreens ‘Making Progress’ on Nursing Home Vaccine Consent
- The Coronavirus Vaccine Could Be the Ultimate Gateway Drug
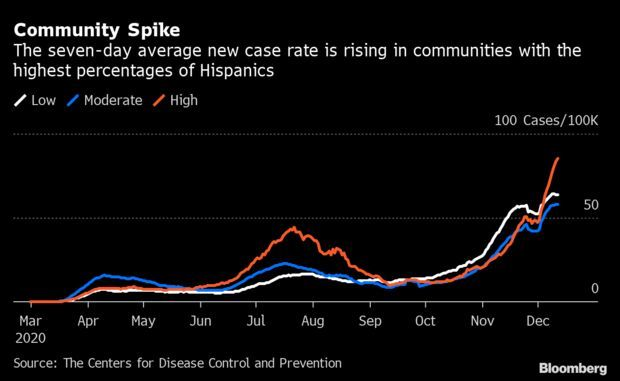
Hispanic Areas Suffer Outsize Pain in Latest Surge: The newest wave of Covid-19 infections is inflicting disproportionate pain on Hispanic communities in the U.S. The seven-day average of new coronavirus cases in heavily Hispanic regions became worse than in those with smaller Hispanic populations from earlier in the month, according to CDC data. The rate has broken a record every day since Dec. 3. There are on average 85 new cases per 100,000 people in counties where the population is almost half Hispanic, 32.6% higher than the national rate. Read more from Nic Querolo.
SCOTUS Orders New Look at N.J. Church Capacity Caps: The U.S. Supreme Court ordered a new round of scrutiny of New Jersey’s capacity limits for churches and synagogues during the coronavirus surge, telling a lower court to take a second look after the justices blocked much stricter caps in New York as violating religious rights. New Jersey rules limit houses of worship to the larger of 10 people or 25% of capacity. Read more from Greg Stohr.
FDA Clears First At-Home, Over-the-Counter Test: The first test for coronavirus that can be performed entirely from home was approved by federal regulators yesterday, and can be acquired without prescriptions. Though availability initially will be limited, the new test and others being developed could make fast coronavirus screenings as accessible as over-the-counter pregnancy tests in the U.S. for the first time. Read more from Emma Court.
More Headlines:
- Biden Urges Staying Home for ‘Extremely Limited’ Inauguration
- N.Y.C. Comptroller Asks SEC to Probe Tyson’s Covid-19 Response
- Psychiatric Hospital Called ‘Tinderbox’ of Covid-19 Infections
Happening on the Hill
Leaders Cite Progress in Talks, No Deal Yet: Speaker Nancy Pelosi (D-Calif.), Senate Minority Leader Chuck Schumer (D-N.Y.), Senate Majority Leader Mitch McConnell (R-Ky.) and House Minority Leader Kevin McCarthy (R-Calif.) met for several hours last night, reporting progress in the talks but with no agreement in hand. They held two rounds of extended negotiations at the Capitol yesterday, trying to reach agreement for a package of aid for businesses and workers struggling through the pandemic’s economic fallout.
“We’re making significant progress and I’m optimistic that we’re gonna be able to complete an understanding sometime soon,” McConnell said as he left the Capitol late last night. “Everybody wants to get a final agreement as soon as possible.” Schumer also said the exchanges had brought progress and “there is a genuine desire to come to an agreement by all four parties.”
The four leaders have been trying to finalize coronavirus aid to attach to the spending bills before funding for federal agencies runs out on Friday at midnight. Both sides have vowed that Congress won’t recess for the holidays without getting both done. Read the latest on negotiations from Bloomberg News.
- One of the two relief bills proposed by the bipartisan group includes language from an earlier Republican proposal that unions and worker advocates warn would stymie the incoming Biden administration’s plan to strengthen workplace safety enforcement to protect workers, Ben Penn reports.
- Related: Covid-19 Aid, Tax Breaks Poised to Hitch Ride on Spending Bill
BGOV Closer Look: Surprise Billing Deal Inches Forward: Patients with health insurance would be protected from surprise medical bills under a discussion draft released by committee leaders Dec. 11, which would use an independent arbitration process to settle payment disputes between insurers and providers. Bipartisan, bicameral leaders of related Congressional committees said they’d work to finalize the measure based on stakeholder feedback and try to attach it to a year-end funding measure. Read more from BGOV analyst Danielle Parnass.
- Meanwhile, Sens. Bill Cassidy (R-La.), Maggie Hassan (D-N.H.) and 25 other senators asked the chamber’s leadership to include the four-committee agreement on surprise billing in any year-end funding bill, according to a press release.
Krishnamoorthi Probes Navarro on Virus Contract: House Oversight and Reform Economic and Consumer Policy Subcommittee Chairman Raja Krishnamoorthi (D-Ill.) called on Peter Navarro, President Donald Trump’s trade czar, to provide information about all pandemic-related government contracts “in which he played any role,” saying his previous involvement in U.S. ventilator procurement “calls into question” his negotiating abilities. Krishnamoorthi cited reports that the ventilator contract resulted in American taxpayers overpaying “by hundreds of millions of dollars.” Find his letter to Navarro here.
Alzheimer’s Treatment: The Senate Finance Health Care Subcommittee meets for a hearing today on Alzheimer’s testing and treatment pipelines, and the cost implications.
House Floor: The House is scheduled to consider the following health care related measures under expedited procedure:
- Mental Health Services for Reentry: Justice Department grants for mental health and crisis stabilization services for incarcerated and recently released individuals would be authorized under S. 3312. The Senate passed the measure by unanimous consent on Nov. 16. On Dec. 14, it adopted, by unanimous consent, S. Con. Res. 52, which would make changes to the measure if the House also adopts it. For more, see the BGOV Bill Summary by Brittney Washington.
- Veterans’ Aid Package: Education benefits, health care, and housing assistance for veterans and their families would be expanded under the Senate-passed version of H.R. 7105. The Senate passed the measure by unanimous consent on Dec. 9. For more, see the BGOV Closer Look by Michael Smallberg, Brittney Washington, Naoreen Chowdhury, and Adam M. Taylor
- Veterans’ Family Caregivers: The Veterans Affairs Department would have to disclose more information about decisions affecting veterans’ family caregivers and could extend their benefits under S. 2216. The Senate passed the bill by unanimous consent on Nov. 17. For more, see the BGOV Bill Summary by Michael Smallberg.
Senate Floor: The Senate by unanimous consent last night passed an amended version of S. 3152, which would require the FCC to incorporate maternal health outcome data into its broadband health maps, as well as an amended S. 2032, to expand research on cannabis and marijuana.
What Else to Know Today
Trump’s $200 Medicare Cards Expected to Ship Jan. 1: The Trump administration expects to begin sending $200 prescription drug discount cards to seniors by Jan. 1, a campaign promise to seniors that Trump was unable to fulfill before losing re-election, a person familiar with the matter said. A White House official described the timeline for shipping out the discount cards to Medicare beneficiaries. Politico reported late yesterday that an industry panel that advises the IRS on administering benefit cards abruptly dropped its opposition to them. Read more from Justin Sink.
High Court Asked to Halt Abortion Pill Deliveries: The Trump administration asked the U.S. Supreme Court to reinstate a requirement that women visit a medical facility before obtaining abortion-inducing pills, seeking to lift a lower-court decision that has allowed delivery by mail amid the pandemic. The filing yesterday renews a request that the court temporarily rejected in October, when it was shorthanded after the death of Ruth Bader Ginsburg, whose seat was filled by the conservative Amy Coney Barrett. Read more from Greg Stohr.
Related: Nevada Covid-19 Restriction on Religious Services Blocked on Appeal
ACA Subsidies Said Secure Despite Tax Return Backlog: The IRS is reassuring people whose tax returns are caught up in its pandemic backlog that they won’t lose the financial assistance they rely on each year to help pay for their health insurance. The IRS, in a statement published yesterday, said the Centers for Medicare and Medicaid Services will delay until 2021 processes to verify those eligible to receive advanced payments of the premium tax credit, and said that people automatically will be re-enrolled in plans they have bought on the federal marketplace. Read more from Allyson Versprille.
More Headlines:
- SCOTUS Again Asked to Weigh Doctors’ Opinions in Fraud Cases
- Pentagon Posts Second Draft RFP for $58B Health Care Contract
- Anti-Doping Agency Parts Ways With Longtime General Counsel
- Southwest Beats COBRA Notice Suit After Labor Dept. Weighs In
- Ohio’s High Court Rejects Patient’s Health Info Disclosure Claim
- Baltimore Seeks Denial, Delay of Review of Family Planning Rule
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.