HEALTH CARE BRIEFING: Houston Approaches ‘Precipice of Disaster’
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Houston-area officials are “getting close” to reimposing stay-at-home orders and are prepared to reopen a Covid-19 hospital established but never used at a football stadium as virus cases expand in the fourth-largest U.S. city.
The announcement by Harris County Judge Lina Hidalgo and Houston Mayor Sylvester Turner yesterday came a day after the Lone Star state recorded its highest one-day tally of new cases since the pandemic emerged.
“We may be approaching the precipice of a disaster,” said Hidalgo, the highest-ranking county executive. “It’s out of hand right now. The good news is it’s not severe out of hand.”
The warnings of a worsening outbreak reinforced alarms sounded by national health officials over the risk of a second wave of infections beyond the initial U.S. hot spots led by New York and New Jersey. Texas has been among the states pushing hardest to ease lockdowns imposed during the first wave of a disease that has killed more than 113,000 Americans.
However, any plan to reinstate local lockdowns may hold little legal authority after Governor Greg Abbott issued executive orders to reopen the state that superceded county and municipal directives, Hidalgo’s spokesman, Rafael Lemaitre, said in an email.
“Hidalgo believes the state is moving too fast to reopen,” Lemaitre wrote just hours after the announcement. Read more from Joe Carroll.
Trump Campaign Asks Rally-Goers to Waive Liability: President Donald Trump traveled to Texas yesterday for two events as that state struggled with a new surge in coronavirus cases. Meanwhile, his campaign is asking people attending his campaign rally in Oklahoma next week to waive liability if they contract Covid-19 — even as he hurtles forward to reopen the country.
An online ticket form for the Trump campaign rally at the BOK Center in Tulsa, Okla., on June 19, tells potential participants that by attending, “you and any guests voluntarily assume all risks related to exposure to COVID-19 and agree not to hold Donald J. Trump for President, Inc.; BOK Center; ASM Global; or any of their affiliates, directors, officers, employees, agents, contractors, or volunteers liable for any illness or injury.” Read more from John Harney.
Related: Schumer Seeks Fauci, Birx Testimony Next Week Amid Cases Spike
Aid Talks & Reopening Efforts
Virus Funds Slow to Reach Hard-Hit Tribes: An administrative bottleneck is slowing the stream of funding aimed at helping American Indian and Alaskan Native tribes deal with the coronavirus pandemic. Tribes are struggling to tap into the federal competitive grants process due to the administrative burden of writing an application, Indian Health Service Director Michael Weahkee said yesterday during a hearing of the House Appropriations Interior-Environment subcommittee. Funding from most agencies are distributed through a grants process.
American Indian and Alaska Native tribes have been hit particularly hard by the pandemic. About 13,800 patients in tribal communities have tested positive for Covid-19, with over half of those cases coming from the Navajo Nation.
The grants have been partially delayed because of a lack of normal contact that the National Institutes of Health and Centers for Disease Control and Prevention have with tribes, Rep. David Joyce (R-Ohio) said at the hearing. Tribes are not accustomed to filling out their grant applications due to that infrequent communication. Shira Stein has more.
Medicaid Providers Fear Delay in Relief: Medicaid providers hit hard by Covid-19 are hailing the Health and Human Services Department’s announcement that long-awaited relief aid is on the way, but they’re concerned it’ll take too long for the funds to arrive. The department said on Tuesday it was directing $15 billion in provider relief aid to Medicaid and the Children’s Health Insurance Program providers who haven’t yet received funding. The agency also plans to distribute $10 billion to safety-net hospitals. Read more from Christopher Brown.
HHS Pressed for Health Stimulus Details: The top Republican and Democrat on the Senate Finance Committee are asking HHS to publish which health-care providers received portions of the about $210 billion in stimulus funding that’s been allocated by lawmakers. “We urge you to expeditiously establish a single, comprehensive and publicly available data source that easily shows the amount of funding received by each provider,“ Chairman Chuck Grassley (R-Iowa) and top Democrat Ron Wyden (Ore.) told Secretary Alex Azar in a letter. Read more from Laura Davison.
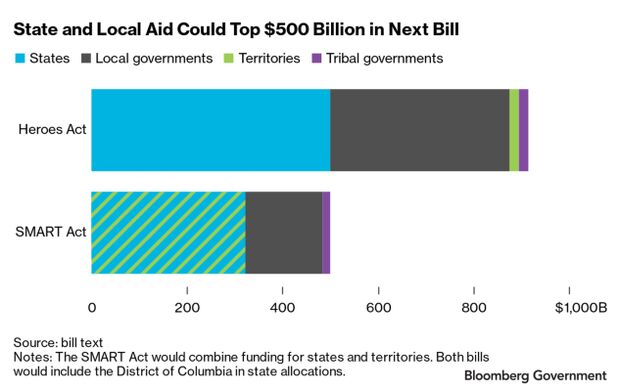
State, Local Aid—BGOV Closer Look: States and cities reeling from the Covid-19 pandemic would get at least $500 billion in additional relief from Congress under two recent proposals. But first, members and the Trump administration will need to hash out their differences over the size of the package, restrictions on states with pre-existing budget shortfalls, and allocations for the District of Columbia, tribal governments, and small towns. Michael Smallberg delves into the relief talks in a BGOV Closer Look.
Related: Biden Releases Plan to Reopen Economy With Major Federal Support
Testing, Treatment & Vaccine Efforts
Extension of FDA Sanitizer Guidance Sought: Senate Republicans including Finance Chairman Chuck Grassley (R-Iowa) unveiled legislation that would extend for two years an FDA policy to aid in the preparation of alcohol-based hand sanitizer products during the coronavirus pandemic. Ethanol and biofuels producers need certainty that their investments made to create hand sanitizers “wouldn’t be lost overnight to a sudden change in policy from FDA,” Grassley said in a statement, Ben Livesey reports.
Moderna Vaccine Trials Moving Fast: Moderna said it had selected a dose for a final-stage clinical trial of its coronavirus vaccine that should begin in July, as the drugmaker moves ahead rapidly with its innovative approach to producing its Covid-19 prevention. The final study, which will include 30,000 people, will be conducted in collaboration with the National Institute of Allergy and Infectious Diseases in the U.S. Its primary goal will be to show the vaccine prevents people from developing symptoms of Covid-19, Moderna said, Robert Langreth reports.
Regeneron Starts Trials of Antibody Cocktail: Regeneron Pharmaceuticals is entering human trials of an antibody cocktail for Covid-19, part of an ambitious clinical-testing plan that could lead to a new treatment option by the end of the summer if all goes well. In a statement, the drugmaker said that it’ll test a two-antibody combination in a wide variety of people, including people already sick with Covid-19, as well as healthy people at a higher risk of being exposed to the virus. Read more from Langreth.
Faulty Virus Antibody Tests Put Pressure on FDA to Demand Better: As researchers learn more about the coronavirus, the FDA’s demands on antibody test makers will change, Commissioner Stephen Hahn said this week at BIO Digital, the first virtual conference of the Biotechnology Innovation Organization. Those changes could lead to more accurate tests. For now, potentially faulty tests can stay on the market while under FDA review. Read more from Valerie Bauman and Jacquie Lee.
Virus Surge Can’t Cause Another Shutdown, Mnuchin Says: Treasury Secretary Steven Mnuchin said yesterday the U.S. shouldn’t shut down the economy again even if there is another surge in coronavirus cases. “We’ve learned that if you shut down the economy, you’re gonna create more damage—medical problems that get put on hold,” Mnuchin told CNBC. “We can’t shut down the economy again.” He said in the event of a resurgence, it’s not necessary to impose restrictions again because Covid-19 testing and contact tracing are improving. Read more from Saleha Mohsin.
Nursing Homes Warned on Seizing Relief Checks: Nursing homes that seize relief payments from residents could face federal enforcement and be blocked from Medicare and Medicaid programs. The Centers for Medicare & Medicaid Services issued the warning today following pressure from lawmakers. The IRS has been issuing checks of up to $1,200 for individuals as part of the CARES Act. Read more from Kaustuv Basu.
More Headlines:
- Novavax: DoD Committed to Negotiate Pact for Added Vaccine Costs
- Outbreak Accelerating in Africa as Cases Reach 200,000, WHO Warns
What Else to Know Today
PE-Owned Hospitals Paid Owners Millions: In the years before coronavirus began stressing the U.S. health system, Leonard Green & Partners extracted over half a billion dollars in debt-funded dividends from its hospital venture, Prospect Medical Holdings. It’s a common tactic for private equity firms like Leonard Green, and it was proving profitable in this case. But with hospitals enduring an unprecedented onslaught, the situation is renewing questions of whether private equity’s playbook conflicts with patient care.
While Prospect says it’s more financially sound than when Leonard Green took over in 2010, its federal care rankings have stayed near the bottom of the scale and in some cases slipped. Lauren Coleman-Lochner and Jeremy Hill have more.
After Enduring Covid, Hospitals Brace for Cancer Onslaught: Across the world, cancer patients face harrowing treatment delays as hospitals try to shield the most vulnerable from contamination risks and salvage overstretched health systems during the pandemic. Routine screenings for breast, cervical and bowel cancer have slowed or stopped altogether, depriving some unsuspecting patients of a chance to tackle the disease early. Read more from Suzi Ring and James Paton.
FTC’s Power Over Pay-For-Delay Deals Tested in Court Fight: A Fifth Circuit fight over the legality of drug patent settlements could test the Federal Trade Commission’s ability to hold drugmakers accountable when they strike deals to keep a generic product off the market.
The U.S. Court of Appeals for the Fifth Circuit heard arguments in an appeal brought by Impax Laboratories against the FTC’s decision to strike down a deal the generic drugmaker made to end patent litigation over Endo Pharmaceuticals’ pain reliever drug. Read more from Valerie Bauman.
More Headlines:
- New Long-Acting Insulin Drug Enters Market With FDA Approval
- FDA Opposes Doctors’ Request to Ease Access to Abortion Pill
- Magenta to Stop Enrollment in Phase 2 Study of MGTA-456
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Zachary Sherwood at zsherwood@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.