HEALTH CARE BRIEFING: CDC Director Denies Warning of Second Wave
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
U.S. Centers for Disease Control and Prevention Director Robert Redfield denied saying to The Washington Post that a second wave of the new coronavirus in the U.S. this winter could be worse than what the country already has experienced.
“I didn’t say that this was going to be worse,” Redfield said at the White House’s coronavirus task force press briefing. “I said it was going to be more difficult and potentially complicated” because of the seasonal flu and coronavirus circulating at the same time.
Redfield was quoted by the newspaper on Tuesday as claiming a resurgence in the virus could coincide with flu season and put a strain on the country’s health-care system. He said yesterday that he was accurately quoted in the article, but that the headline lacked context.
“There is a possibility that the assault of the virus on our nation next winter will actually be even more difficult than the one we just went through,” Redfield told The Washington Post. “When I’ve said this to others, they kind of put their head back, they don’t understand what I mean.”
Redfield’s comments to the Post threatened to create a political problem for his boss, President Donald Trump, whose November re-election could be imperiled by concern over a resurgence of the coronavirus. Earlier yesterday, Trump said Redfield was “totally misquoted” by CNN, which summarized the Post’s report.
At yesterday’s briefing, Trump said the U.S. will be ready when the fall flu season arrives, even if there are new flare-ups of Covid-19. ‘From everything that I have seen, it can never be like anything we witnessed right now,” Trump said. “If they combine and come together, it’s not great, but we will not go through what we went through for the last two months.” Read more from Drew Armstrong.

More Headlines:
- Trump Says Coronavirus ‘Might Not Come Back at All’ in Fall
- Gulf Widens Among States Over When to End Coronavirus Lockdown
- Trump Says Georgia Governor’s Decision to Re-Open Is ‘Too Soon’
Health Aide Says He Was Fired Over Trump-Touted Drugs
A top federal health official who was helping lead efforts to find a coronavirus vaccine said he was removed from his post because he insisted on limiting the use of a drug Trump has pushed as a Covid-19 treatment despite little clinical evidence it works.
Rick Bright was abruptly pushed out of his position as director of the Biomedical Advanced Research and Development Authority on Tuesday and given a smaller role at the National Institutes of Health. BARDA is helping drugmakers develop a vaccine for the novel coronavirus.
The drugs Trump has touted, hydroxychloroquine and chloroquine, “clearly lack scientific merit,” Bright said in a statement released by his lawyers yesterday. “I rightly resisted efforts to provide an unproven drug on demand to the American public,” he said. “Sidelining me in the middle of this pandemic and placing politics and cronyism ahead of science puts lives at risk.”
Trump, asked about Bright at the White House briefing yesterday, said “I never heard of him. When did this happen?” He then added: “I don’t know who he is.”
Bright’s former office had received an emergency authorization last month from the FDA to use hydroxychloroquine and chloroquine in the national stockpile for hospitalized Covid-19 patients. BARDA oversees the stockpile. During an April 10 interview with Bloomberg, Bright emphasized the need for conducting rigorous scientific research even in the midst of a pandemic.
“It is very difficult to do development and conduct research in the middle of a pandemic outbreak,” he said on a call that focused on some of BARDA’s drug research in Covid-19 treatments. Anna Edney and Robert Langreth have more.
Related: Fauci: Not Concerned About Health Officials’ Ability to Speak Up
Top Democrats expressed concern over the ousting of Bright:
- House Appropriations Labor-HHS-Education Subcommittee Chairwoman Rosa DeLauro (D-Conn.) called Bright’s removal “authoritarian” and “anti-science,” saying it may “have long-lasting and deadly effects for the American public.” She cited a “forthcoming request” by Bright for the HHS inspector general to probe the politicization of BARDA’s work and is “wholeheartedly” on board.
- Senate Health, Education, Labor, and Pensions Committee ranking member Patty Murray (D-Wash.) said “a global pandemic is not the time to shuffle personnel, or contradict and remove experts for wanting to do their job well,” and called Bright’s removal “incredibly disturbing” while adding she will be pushing for answers.
- Separately, Democratic Sens. Mark Warner (Va.) and Tim Kaine (Va.) sent a letter to the FDA yesterday expressing their concerns of shortages in hydroxychloroquine and chloroquine after Trump touted the medications. The pair has “heard directly from health care providers and Virginians who depend on these lifesaving drugs and fear losing access to them,” they said in a statement, urging the FDA “work to ensure that patients who have long depended on these drugs continue to have access to them.”
Stimulus Talks & Financial Woes
Masked Lawmakers to Vote in Person to Pass Stimulus: Welcome to legislating in the pandemic era: U.S. House members in masks, disinfectant, silent committees and hundreds of billions in deficit spending.
The House plans to convene at 10 a.m. Thursday to give final passage to an interim rescue plan to bolster a staggered American economy. It will be the first time lawmakers have gathered in such a large group since March 27, when the last stimulus plan was approved, and they will be casting a roll-call vote under extraordinary circumstances to match the times.
Unlike the Senate, which was able to pass the latest emergency package in a quick voice vote with only a handful of senators present, the possibility of an objection from either party means at least half of the 429 current House members must venture to Washington despite the risks from the coronavirus pandemic. Read more from Erik Wasson and Billy House.
House Set to Pass New Stimulus: With the House poised to give final passage to a package of interim pandemic relief funds this afternoon, Congress and the Trump administration are already turning their focus on the next round of stimulus for a stalled economy. The legislation the House will take up was passed by the Senate on Tuesday. Read more from Laura Litvan, Erik Wasson, and Billy House.
- BGOV Bill Summary: H.R. 266, SBA Loans & Hospital Aid
- BGOV OnPoint: Food Aid Expanded During Covid-19 Outbreak
U.S. to Award Health Providers $20 Billion: The federal government plans to distribute another $20 billion to health providers whose businesses have been struck by the pandemic, adding to $30 billion that was already distributed. The money is part of the $100 billion set aside for hospitals, physician practices and other health-care providers in the $2.2 trillion stimulus package meant to help people, businesses, and organizations amid the crisis. While some hospitals are inundated with Covid-19 patients, many are struggling financially after canceling elective procedures and office visits that are big money-makers for the systems.
The initial $30 billion was distributed based upon providers’ Medicare fee-for-service revenue, a simple calculation that let the government send the money quickly. But some hospitals said it resulted in hospitals that need the funding most getting shortchanged in the initial round. Drew Armstrong has more.
Rural Hospitals Get HHS Relief: Small rural hospitals and telehealth education centers will get nearly $162 million to prevent, diagnose, and treat Covid-19, the HHS said yesterday. The funding will be divided among 1,779 rural hospitals to help them respond to the pandemic and among 14 telehealth resource centers, which help rural hospitals in offering telehealth services, the Health and Human Services Department said. HHS Secretary Alex Azar said the funds would help to expand telehealth, procure personal protective gear, and boost testing capacity. Read more from Shira Stein.
- Meanwhile, some hospitals complained that the new portal HHS set up to help distribute the money is having troubles, and some can’t submit their information. America’s Essential Hospitals, an industry group representing 300 hospitals and health centers, urged Azar to extend the deadline until the technical glitches are fixed.
Uncertainty on Entitlement Forecasts: Medicare’s trust fund will keep it afloat until 2065—13 years longer than forecast last year—but how the pandemic may affect that remains to be seen, according to an annual report. The report from the Social Security and Medicare Boards of Trustees also anticipates that Social Security can continue paying full benefits until 2034, the same as projected last year. Both programs will see costs exceed GDP growth through the mid-2030s as baby boomers continue to age into retirement. Andrew Childers has more.
More Headlines:
- Pot Shops Need Federal Aid to Weather Virus Crisis, Senators Say
- Tiny Drugmaker Joins 3M, J&J in $7.2 Billion U.S. Virus Windfall
- Leaders Urged to Include Anti-Surprise Billing Language in Next Bill
State-Level Testing & Tracing
Health Departments Finally Get Software to Track Cases: As states and cities begin to think about reopening their economies, the public-health workers who will be responsible for keeping Covid-19 from spreading face an unprecedented challenge: how to track and isolate thousands of people exposed to a virus that spreads with remarkable efficiency. For years, state and local health authorities have relied on Excel, paper, emails and phone calls to battle measles outbreaks, sexually transmitted diseases and other infections.
But, with more than 800,000 confirmed cases of Covid-19 in the U.S., that’s not enough. A new software tool called Sara Alert, developed by a federally funded nonprofit, aims to solve that arduous data-management problem. “The concept here is quickly find the brush fire and extinguish the brush fire before you have a forest fire,” said Paul Jarris, chief medical adviser at Mitre, the research group that developed the software. Read more from John Tozzi.
- Meanwhile, New York state is building a “tracing army” to track the origin of individual coronavirus cases and reduce the spread so the state can focus on re-opening, Gov. AndrewCuomo (D) said yesterday. He unveiled plans to work with New Jersey and Connecticut to increase contact tracing. Keshia Clukey has more.
- New York City officials also plan to enlist thousands of health-care workers next month to conduct hundreds of thousands of diagnostic tests per day, and isolate anyone found to be carrying the disease. The plan hinges on the city’s still-unmet capacity to test its residents, Mayor Bill de Blasio (D) said at a press briefing yesterday. Read more.
California to Expand Testing: California Gov. Gavin Newsom (D) said he had a “very good” conversation yesterday with Trump, who agreed to send the state a minimum of 100,000 swabs needed to boost testing for the novel virus. Another 250,000 are expected next week, he said. Newsom is aiming to sharply increase testing for the coronavirus as a step toward re-opening the world’s fifth-largest economy. California currently has capacity to test 16,000 per day and expects to reach 25,000 by the end of the month, with an ultimate goal of 60,000 to 80,000, he said at a press briefing. Read more.
Puerto Rico Reviews Virus Testing Scandal: Puerto Rico’s financial oversight board is looking into an agreement by Gov. Wanda Vazquez’s administration to buy coronavirus testing kits from inexperienced local companies that charged more than three times as much as a rival bidder. The oversight board received some 1,200 pages of documents tied to the contract on Monday, after several requests to the administration, according to board spokesman Matthias Rieker. Read more from Michelle Kaske.
Outbreak-Related HHS Rule Changes Now Online: The Trump administration is making it easier for state and local health-care authorities dealing with Covid-19 to sort out what they can and can’t do. It’s putting all guidance and regulatory actions—and there are a lot—in one place. The Centers for Medicare & Medicaid Services yesterday released an online coronavirus “Workforce Virtual Toolkit” for health-care decision-makers that includes information on funding opportunities, liability protections, and workforce training.
The White House has taken a number of actions to bolster the health workforce to treat Covid-19 patients, including allowing medical practitioners to practice at the top of their license and across state lines and expanding telehealth services covered by Medicare. In one example, the administration has specific guidelines for different types of health-care providers on how they could expand the scope of their practices to respond to the coronavirus. Read more from Sara Hansard.
More Headlines:
- U.S. Confirmed Cases Rise 3.1%, Compared to 4.4% Last Week
- Almost 90% of Covid-19 Patients on Ventilators Died, Study Finds
- U.S. Coronavirus Death Toll Could Double in Prisons, ACLU Says
- Indian Health Service Seeks Mobile Critical Care Teams for Virus
- Fifth Circuit Stays Injunction on Texas Prisons in Covid-19 Suit
What Else to Know
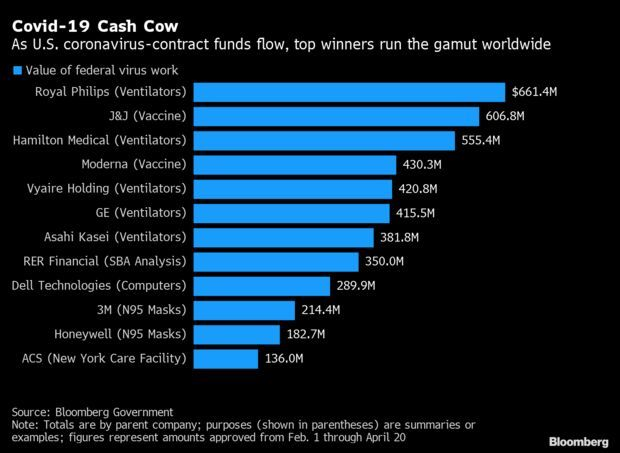
Top Winners From Virus Contracts: The gusher of U.S. government contracts aimed at fighting the coronavirus reached $7.2 billion this week, and the money is benefiting big and small companies across the globe. The top dozen winners shown here are dominated by American businesses, but also include ventilator-makers from the Netherlands and Japan (Royal Philips and Asahi Kasei), as well as a Spanish construction-services outfit, ACS, hired to construct a health-care facility on Long Island in New York. Several closely held firms are also on the list, Phil Kuntz and Ryan Beene report.
WHO Warns Protests Could Fuel Outbreak: The director of the World Health Organization said that protests would worsen the coronavirus crisis. “Protests and gatherings in the middle of the pandemic will not help,” Tedros Adhanom Ghebreyesus said at a press briefing yesterday. “It will only fuel the outbreak.” Government leaders need to win the trust of citizens and engage with them in two-way dialogues to manage social pressures, he added. His comments come after protests against government lockdowns in several U.S. states. Read more.
Arkansas Can Restrict Surgical Abortion: Abortion providers in Arkansas may not perform surgical abortions while an emergency order requiring that elective surgeries be postponed due to the coronavirus pandemic is in effect, the Eighth Circuit said today. A lower court’s temporary restraining order barring the state health department from enforcing the directive against abortion providers was improper because the lower court did not follow the appropriate framework for deciding when a state may constrain constitutional rights in response to a public emergency, the Eighth Circuit said, Mary Anne Pazanowski reports.
More Headlines:
- GSK, Teva Get Class Certification Vacated in Generic Drug Suit
- Biogen Cut at Raymond James on Risk FDA Rejects Alzheimer’s Drug
- Biogen Alzheimer’s Drug Still Divides Wall Street After Delay
- Claims Against Texas County Over Drug Suicide Partially Revived
- Immunomedics Surges After Breast Cancer Drug Gets FDA Nod
- Colorado Pharmacy Board Must Give DEA Patient-Identifying Info
- Banner Health $8.9 Million Data Breach Settlement Gets Court Nod
- Earth Day Highlights Link Between Climate Change and Coronavirus
- Temperature Checks and Constant Anxiety in Pandemic’s City Zero
- Texas Lets Abortions Resume After Clinics Swear Off Masks, Gowns
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Zachary Sherwood at zsherwood@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.