HEALTH CARE BRIEFING: Vaccine Success Hinges on Schools, Offices
By Brandon Lee
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Employers and schools will do more in the effort to get Americans to roll up their sleeves for a Covid-19 shot than a universal vaccination mandate, legal scholars and public health officials said.
The first vaccines could be available as early as Dec. 11 or 12 in the U.S. after partners Pfizer and BioNTech requested emergency authorization of their vaccine. Moderna is likely not far behind on its submission to the Food and Drug Administration.
With vaccines produced at record speed, the next challenge is to make sure enough people receive it to bring an end to the pandemic that’s killed more than 256,000 Americans. About 60% to 70% of the U.S. population—200 million Americans—would need protection from the coronavirus in order to reach herd immunity.
It’s “vanishingly unlikely” that states will require universal vaccination even though they have that power, Lawrence O. Gostin, director of Georgetown University’s health law institute, said.
Where a state mandate could cause a political backlash and discourage vaccinations, schools and employers are more likely to drive demand for inoculations. As long as they don’t single out any groups based upon a pre-existing condition or other disability, requiring vaccinations for re-entry would be legal, Gostin said.
Workplace mandates tend to push vaccine rates up over 90%, even absent strict enforcement, said Dorit Rubinstein Reiss, a professor at the University of California Hastings College of the Law, who writes about school mandates and other vaccine issues. That would be particularly important when nearly half of the American public said they wouldn’t take a vaccine.
“If it’s just sitting on the shelf and no one’s willing to take it, then we may as well not have it,” Dial Hewlett, an Infectious Diseases Society of America fellow, said. Read more from Jeannie Baumann.
More on the Pandemic
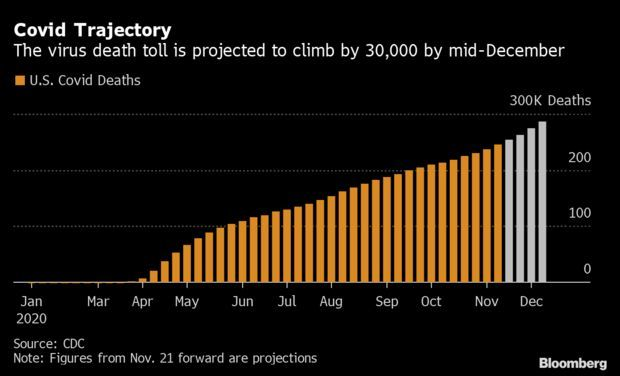
CDC Sees 30,000 More Deaths by Christmas: After a week that shattered daily case, testing, and hospitalization records, the trajectory of the coronavirus is slated to steepen in the U.S. Covid-19, which has killed over 256,000 Americans so far, is on track to claim another 30,000 lives by mid-December, a new forecast from the Centers for Disease Control and Prevention shows. The agency’s model shows weekly cases and deaths both increasing weekly through all of December, the maximum range of the CDC’s projection.
Other models stretch further into the future and paint a picture of what the disease may look like when President-elect Joe Biden inherits the crisis Jan. 20. Data from the Institute for Health Metrics and Evaluation at the University of Washington in Seattle show daily deaths peaking in early January on the current path, at around 2,560. That would imply a death toll of more than 387,000 by Inauguration Day.
The institute’s model suggests that watered-down social distancing mandates would push the peak to early February at over 5,600 deaths each day. The university’s forecasts don’t factor in how a vaccine may slow the spread of the coronavirus, but researchers anticipate adding that to their forecast in the coming weeks. Read more from Nic Querolo.
- Meanwhile, airlines set a new pandemic record for U.S. passengers over the weekend, as Thanksgiving spurred travel despite government warnings. A total of 1,047,934 travelers went through airport screenings, the most since the coronavirus torpedoed demand for flights in mid-March, data compiled by the Transportation Security Administration show. Read more from Alan Levin.
Hospitals Urge Biden to Extend Emergency Declaration: The industry group representing for-profit hospitals called on the upcoming Biden administration to extend the public health emergency and its waivers next year. The Federation of American Hospitals said yesterday it will be “crucial” to keep in place for the duration of the public health emergency some of the “foundational steps that Congress and the current Administration have taken over the past eight months to provide support to individuals, states, businesses, and health care providers.”
Through various new laws and executive actions under the emergency declaration, hospitals can screen patients for Covid-19 offsight to help avoid outbreaks of the coronavirus within their buildings, get reimbursed for telehealth services at higher-than-normal rates and make other changes to how they treat patients, Alex Ruoff reports. Read the letter here.
More on Biden’s Agenda:
- Biden Wins Access as Agency Gives Him Transition Authority
- Biden Plans to Tap Yellen for Treasury to Deliver Pandemic Relief
- Key to Biden OSHA Transition, Virus Rule Is Naming Deputy First
Regeneron Drug to Roll Out 30,000-Dose Shipment: Thirty thousand doses of Regeneron Pharmaceuticals’s antibody cocktail will be shipped later today, after the experimental Covid-19 treatment got an emergency authorization from U.S. health regulators. Health and Human Services Secretary Alex Azar detailed plans to distribute the therapy drug at a news conference yesterday. President Donald Trump received the treatment when he was hospitalized with the virus and credited it for his recovery. Read more from Robert Langreth and Emma Court.
AstraZeneca Shot Hints at Preventing Severe Cases: The leader of the U.S. government’s coronavirus vaccine program said AstraZeneca found that 16 participants who received a placebo in its clinical trial contracted severe Covid-19, a sign that the shot could block the worst cases of disease. The British drugmaker and its partner, the University of Oxford, said earlier yesterday that none of the trial participants who received the vaccine had become severely ill, and that none of the patients in that group were hospitalized.
“That’s very important,” said Moncef Slaoui, chief scientific adviser to Operation Warp Speed, in an interview with Bloomberg News. “It’s exciting.” Read more from Riley Griffin.
Virus Persistence Leads Parties to Half Measures: In the pandemic’s first months, the split between Republican and Democratic governors was vast. Most Republicans allied with Trump refused to impose basic public-health restrictions like mask-wearing, while Democrats closed huge swaths of their states’ economies. But today, many find themselves implementing very similar, often half-hearted, measures, a bipartisan weariness aimed less at deep change than easing the strain on medical systems and buying time until a vaccine arrives. Read more from Richard Chess and Christopher Palmeri.
Merck Buys OncoImmune to Gain Covid-19 Drug: Merck has agreed to acquire privately held biopharma firm OncoImmune for an upfront payment of $425 million to gain a potential therapy for severe Covid-19. OncoImmune recently announced positive interim findings from a late-stage study evaluating its lead candidate for the treatment of those with a severe case of the disease. The therapy is designed to tamp down inflammation caused by virus-induced damage to cells, an underlying cause of complications in hospitalized Covid-19 patients. John Lauerman and Riley Griffin have more.
More on the Surge:
- U.S.’s Virus Hospitalizations Skyrocket to 12.5%; Most Since April
- New York Opens Overflow Ward on Staten Island as Covid-19 Soars
- Meatpacking Link Found in Up to 8% of Early U.S. Covid-19 Cases
What Else to Know
UnitedHealth Cites High Court in Billing Practice Lawsuit: UnitedHealth Group is fighting a proposed class action challenging how it moves money among the thousands of health plans it administers, telling a Minnesota federal judge that there’s no injury giving plan participants standing to sue. Participants fighting UnitedHealth’s practice of “cross-plan offsetting” under the Employee Retirement Income Security Act can’t show that their own benefits have been or will be diminished, the insurer said, citing the U.S. Supreme Court’s June 1 decision in Thole v. U.S. Bank NA. Read more from Jacklyn Wille.
More Headlines:
- Planned Parenthood Patients Lose Texas Medicaid Program Appeal
- PhRMA Names Debra DeShong as Vice President of Public Affairs
- Cigna CEO, Board Hit With $1.85 Billion Suit Over Anthem Deal
- Nearly All Uses of Solvent Pose Cancer and Other Risks, EPA Says
- Viatris Gets Tentative FDA Approval for Pediatric Dolutegravir Pill
- Doctors’ Cancer Drug Caches Take Hit Under Foreign Pricing Rule
- Pharmacy Group Seeks to Block U.S. Drug Imports From Canada
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.