HEALTH CARE BRIEFING: Trump Pressed to Levy National Virus Rules
By Brandon Lee
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President Donald Trump is coming under increased pressure to impose national rules as the U.S.—already with the world’s highest coronavirus death toll by far—struggles to contain the spread of the coronavirus.
The Trump administration has largely avoided levying strict rules or a national plan, leaving many of the decisions to industry or state leaders. That approach is exacerbating shortages of personal protective equipment at hospitals, and it could complicate distribution of future vaccines, experts say.
“It would be much better if we acted as a country,” said Ashish Jha, director of the Harvard Global Health Institute. He recommended that the administration mandate outlining when Americans should don masks nationwide to slow the transmission of the Covid-19 virus. The White House has said it will leave mask requirements up to states.
The debate over whether and how to impose a nationwide mandate will be on display when Anthony Fauci, the U.S government’s top infectious disease expert, and Robert Redfield, director of the Centers for Disease Control and Prevention, testify later today to the House Select Subcommittee on the Coronavirus Crisis.
Democratic leaders said they want to require the White House to lay out a plan for distributing an eventual coronavirus vaccine. The next stimulus must have a “comprehensive, end-to-end national vaccines plan that addresses supply chain issues, health disparities, vaccine confidence and more,” Patty Murray (D-Wash.), top Democrat on the Senate Health, Education, Labor, and Pensions Committee, said.
House Energy and Commerce Chairman Frank Pallone (D-N.J.) made the case to the chamber’s Democratic Caucus this week that the next stimulus package should have a national testing and tracing plan and called for a “medical supply response czar” to handle supply chain issues, two Democratic aides say. Read more from Alex Ruoff.
Redfield and Fauci will inventory measures taken to improve testing and develop therapeutics and vaccines, according to the testimony. But the prepared remarks don’t discuss what many health experts say is needed and the hearing is on: A national strategy to contain the coronavirus to replace the current state-by-state piecemeal effort. Covid-19 “is the most significant public health challenge to face our nation in more than a century,” the officials said in a joint statement that was released by the committee. Read more from Emma Court and Robert Langreth.
Related: Fauci, Redfield, Giroir Testimony Posted Ahead of House Hearing
On Lawmakers’ Radars
House Appropriations: House Democrats aim to pass a spending package including six full appropriations bills today (H.R. 7617) that includes funding for programs under the Health and Human Services Department.
The chamber will also vote on an amendment (No. 219) from Rep. Rick Allen (R-Ga.) that would reduce the total amount of funding in the Labor-HHS-Education portion of the bill by 5%.
Lawmakers voted on the floor yesterday to adopt amendments that would prevent the military from banning transgender people from service, and call on several agencies to determine and annually update a list of 300 to 400 medications that would be needed in a public health emergency, Jack Fitzpatrick reports.
- BGOV Closer Look: Amendments to Second FY 21 Minibus, H.R. 7617
- BGOV Bill Summary: H.R. 7614, Fiscal 2021 Labor-HHS-Education Funding
The Trump administration threatened a veto of the House appropriations bill, according to a White House’s Office of Management and Budget statement. It cited language in the measure that it says prohibits the HHS Office for Civil Rights from enforcing a “conscience rule” that allows health-care providers to refuse certain services if they have religious or moral objections, among other provisions. Read the White House statement here.
Congress Heads for Weekend With No Deal on Virus Relief in Sight: The Senate left Washington for the weekend after a fourth day of negotiations yielded little substantial progress on narrowing differences between Republicans and Democrats on a plan to bolster the coronavirus-ravaged U.S. economy.
House Speaker Nancy Pelosi (D-Calif.) and Senate Democratic leader Chuck Schumer (D-N.Y.) rejected a temporary extension of lapsed supplemental unemployment insurance proposed by President Donald Trump’s Treasury Secretary Steven Mnuchin and Chief of Staff Mark Meadows. “On certain issues we made progress, on certain issues we’re still very far apart,” Mnuchin said after leaving a two-hour meeting in Pelosi’s office Thursday night. He said they would continue talking Friday and Saturday “as long as it takes to get this done.” Read more from Daniel Flatley, Laura Litvan and Steven T. Dennis.
Panel Backs Allowing Americans to Sue China Over Virus: The Senate Judiciary Committee approved a measure on a 13-9 vote that would let American citizens sue China in federal court for damage done to small businesses and families due to the pandemic, according to Chairman Lindsey Graham (R-S.C.). “To those who have suffered from the coronavirus, your suffering is directly related to the outrageous behavior of the Communist Party of China,” Graham said, Elizabeth Elkin reports.
- Separately, Moderna was targeted by Chinese government-linked hackers earlier this year in an attempt to steal data, Reuters reported, citing a U.S. security official tracking Chinese hacking activity. “Moderna remains highly vigilant to potential cybersecurity threats,” a spokesman told Reuters. The company is making significant progress on a Covid-19 vaccine candidate. Read more from Reuters.
Research & Treatment Efforts
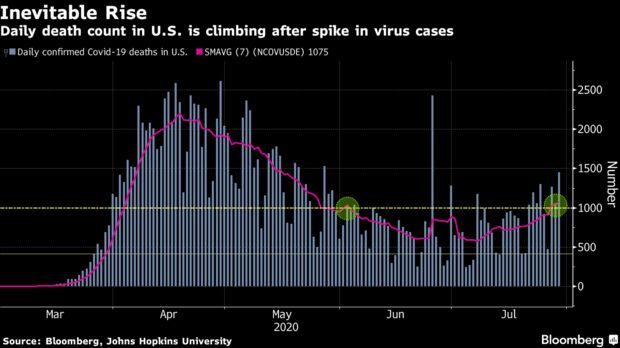
Over 1,000 Americans Die of Covid a Day: The daily count of confirmed Covid-19 deaths in the U.S. is showing how the world’s largest economy is struggling to contain the pandemic. The seven-day average of fatalities topped 1,000 for the first time since early June on Monday and has stayed above that level, following last month’s spike in infections. And yesterday, the nation marked another grim milestone: More than 150,000 Americans have succumbed to the virus.
Trump Urges Americans to Give Plasma: Trump yesterday asked Americans who have recovered from Covid-19 to donate their plasma at local blood banks to help those who contract the coronavirus potentially get better faster. Trump told those who have had the virus that they “have something very special,” and asked them to donate “as soon as you can.” Plasma from people who recovered from Covid-19 is known as convalescent plasma, and it contains antibodies that can help others fight the virus. Read more from Anna Edney and Jennifer Jacobs.
- Hospitals can track the use of Gilead’s remdesivir and convalescent plasma for treating Covid-19 patients starting Aug. 1, the Centers for Medicare and Medicaid Services announced yesterday. Codes will let Medicare and other U.S. insurers track the use of these therapies. That evidence will provide critical information on the drugs’ usefulness, Fawn Johnson reports.
- Related: Gilead Plans to Make 2 Million Remdesivir Doses This Year
Johnson & Johnson Eyes Large-Scale Trials: Johnson & Johnson wants to start Phase 3 Trials of its Covid-19 vaccine in September, the company said yesterday in a statement. The drugmaker’s experimental candidate protected a handful of primates with a single shot in an earlier study, prompting the company to begin trials in humans this month. A study published in Nature showed that its Covid-19 candidate elicits a strong immune response. Read more from Rebecca Smith.
- Meanwhile in the U.K., nearly 10,000 people were given a Covid-19 vaccine candidate from AstraZeneca and Oxford University in a major step toward finding a shot that will help control the pandemic. AstraZeneca is also well on its way to administering shots to 5,000 volunteers in a late-stage trial in Brazil and may scale up the size of its studies there, CEO Pascal Soriot said. Read more from Suzi Ring.
More Headlines:
- Hahn Says Hydroxychloroquine Decision Up to Doctor, Patient
- CDC Sees Possible Safety Benefits to Expanding Absentee Voting
- Redhill Biopharma Begins Global Phase 2/3 Study for Covid-19
- DeWine Eyes Halting Ban of Trump-Touted Hydroxychloroquine
- New York Sets Aside $30 Million to Fund More Contact Tracing
- Florida Reports Record Deaths for Third Day; Arizona Toll Rises
- Nearly Half-Million Teachers Lack Health Insurance, Survey Finds
- Covid-19 Contracts Worth $18 Billion Largely Noncompetitive
- New York City Steels Itself for Virus to Return in the Fall
- Tobacco-Based Covid Vaccine May Start Clinical Trials in Weeks
What Else to Know
Ban on FDA Abortion Pill Dispensing Rule Stands: The Food and Drug Administration can’t stop doctors from using mail or a delivery services to send women a pill to induce abortions while it appeals an order blocking its in-person dispensing requirement, a federal court in Maryland said yesterday. The agency didn’t show it was likely to win on merits or be irreparably harmed by the order prohibiting it from requiring women to visit a hospital, clinic, or doctor’s office to get mifepristone during the coronavirus pandemic, the U.S. District Court for the District of Maryland said. Read more from Mary Anne Pazanowski.
More Headlines:
- Smithfield and OSHA Settle Suit Over Health Inspection Records
- Nevada OSHA Takes Lead Enforcing Workers’ Covid Protections
- Seegene Says FDA Expands Allplex Ncov Assay Emergency Use
- U.S. Visa Rules Trap Migrant Workers in Virus-Infested Dorms
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Zachary Sherwood at zsherwood@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.