HEALTH CARE BRIEFING: Health Sector Groups Call for Gun Control
By Brandon Lee, Alex Ruoff and Jeannie Baumann
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Some of America’s most-influential health industry groups are throwing their support behind congressional efforts to curb gun violence. The American Medical Association, the top lobby for physicians, wrote to the Democratic and Republican heads of the House Judiciary Committee on Thursday in support of H.R. 7910.
“Gun violence is a public health crisis,” James Madara, executive vice president of the AMA, wrote in a letter sent to the panel the same day it was marking-up the bill. “As with other public health areas, evidence-based interventions are needed for reducing deaths and injuries. As physicians, our mission is to heal and to maintain health.”
Other groups called broadly for Congress to act. The head of the American Hospital Association, Rick Pollack, urged Congress to “act quickly” to tackle gun violence, while the head of the Federation of American Hospitals, Chip Kahn, said he hopes bipartisan talks will “result in legislation that will have a real impact on the scourge of gun violence.”
These groups spend millions of dollars each year on both lobbying and campaign donations to influence members of Congress, and are often consulted as experts on public health issues. Recently doctors’ groups in particular have become increasingly vocal on the issue of gun violence, Alex Ruoff reports. Read the letter here.
- Meanwhile, President Joe Biden called for a ban on sales of assault weapons and high-capacity magazines, among other measures, pleading with Congress in a prime-time address from the White House on Thursday to toughen gun laws following a spate of mass shootings, Justin Sink and Jennifer Jacobs report.
The Coronavirus Pandemic
Vaccine for Young Kids to Get Green Light in Weeks: Children under age 5 are poised to be able to receive Covid vaccines as soon as the week of June 21, Biden’s Covid-19 czar said, if regulators ultimately authorize the shots. “We expect that vaccinations will begin in earnest as early as Tuesday, June 21, and really roll on throughout that week,” Ashish Jha said at a briefing on Thursday.
A Food and Drug Administration advisory panel will meet June 15 to consider whether to clear vaccines by Moderna and Pfizer-BioNTech for use in younger children. The FDA will then make its own decision, followed by the Centers for Disease Control and Prevention.
The Biden administration continues to call on Congress to pass a new round of pandemic funding, warning that the government will otherwise run out of vaccines and treatments and also risks a collapse of Covid-19 test production. Read more from Josh Wingrove and Jennifer Jacobs.
Pfizer Pill Access Stymied by ‘Vague’ Prescribing Guidance: High-risk Americans are having a tough time getting Covid-19 antiviral pills as prescribers grapple with limited guidance on who is eligible and how many are available, specialists say. Over 2,500 Test-to-Treat sites are set up across the US where patients can get tested and prescribed Pfizer‘s Paxlovid or Merck’s molnupiravir by a health provider at a single location. But some providers are reluctant to prescribe them due to obscure guidance. Read more from Celine Castronuovo.
Staff Gaps to Cost Care Centers $19.5 Billion: US staffing shortages amid the Covid pandemic will potentially cost nursing and rehabilitation facilities as well as home-health agencies $19.5 billion this year. That’s the conclusion of a study by consulting firm Oliver Wyman. Read more from Lauren Coleman-Lochner.
More Headlines:
What Else to Know Today
Senators Pressed to Pull Diagnostics Reform From User Fee Push: More than 90 groups, including a number of academic laboratories, are calling on Senate health leaders to pull diagnostics reform legislation from its FDA user fee package (S. 4348) in a letter provided to Bloomberg Law’s Jeannie Baumann. The Senate Health Education Labor and Pensions Committee plans to mark up the user fee bill June 8.
The FDA has historically regulated commercially developed tests as medical devices while exerting enforcement discretion over tests developed and manufactured in a single laboratory. The new measure, known as the VALID Act, would create a new category of medical products known as in-vitro clinical tests that the FDA would oversee regardless of who made the test. The FDA would then calibrate the level of FDA oversight to the level of risk.
Proponents of the bill say the changes are necessary to ensure the complex tests that can scan millions of genomic sequences at one time are both accurate and useful. But the letter said the costs associated with VALID would force labs to consolidate their testing, disrupting access to the tests. Read the letter here.
Abortion Rights Support Highest Since 1995: Support for abortion rights has hit the highest level in decades. A new Gallup poll found 55% of Americans identify as “pro-choice,” the highest level since 1995. Those calling themselves “pro-life” — 39% in the latest poll— was at the lowest level since 1996. Kelsey Butler has more.
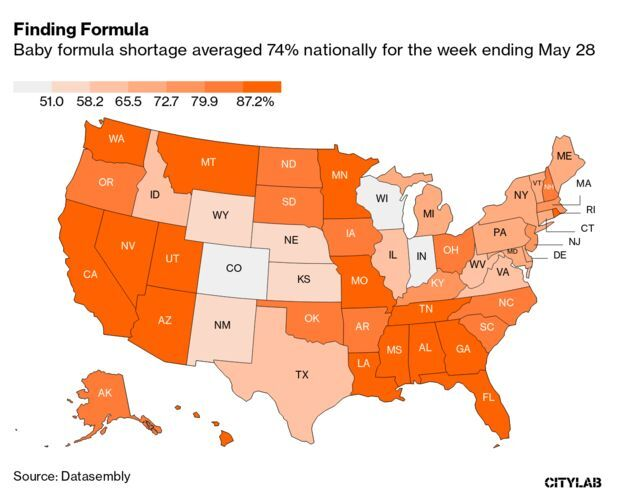
Formula Shortages Hit 74% Despite Biden Action: The baby formula shortage continued to worsen last week despite actions from the Biden administration to ease constraints, according to retail tracking data. Out-of-stock rates climbed to 74% nationally for the week ending May 28, according to data on 130,000 stores followed by Datasembly. Martine Paris has more.
- Meanwhile, a Department of Health and Human Services watchdog will review FDA’s actions leading up to the infant formula recall at Abbott Laboratories’s Sturgis, Mich., facility in February 2022, the department said. The move seeks to determine whether the FDA followed proper procedures for conducting inspections of the manufacturing facility and for overseeing Abbott’s infant formula recall, Maria Luiza Rabello reports.
More Headlines:
- November Elections Put Drug Pricing Back on the Table: Analysts
- FTC Sues to Block RWJBarnabas, Saint Peter’s Healthcare Merger
- Maker of Covid-Test Swabs Loses Bid for Immunity in Patent Suit
- Illumina’s DNA-Data Security Threat Prompts Warning From FDA
- Washington Top Court Rejects Direct Patient Drug Warnings
To contact the reporters on this story: Brandon Lee in Washington at blee@bgov.com; Alex Ruoff in Washington at aruoff@bgov.com; Jeannie Baumann in Washington at jbaumann@bloombergindustry.com
To contact the editors responsible for this story: Michaela Ross at mross@bgov.com; Giuseppe Macri at gmacri@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.