HEALTH CARE BRIEFING: Democrats Target Drug Prices for BBB Unity
By Brandon Lee
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
A key Senate Democrat says his party’s signature domestic spending legislation can be revived around two key areas: reducing prescription drug costs and boosting clean energy tax credits.
Senate Finance Chairman Ron Wyden (D-Ore.) told reporters yesterday that Democrats agree broadly on expanding access to health care, empowering the government to negotiate with drugmakers and offering tax incentives for clean energy. Senate Democrats hope to clear a package soon built around these policies, he added. “The reality is there is a lot of common ground right now,” Wyden said.
The Build Back Better Act has been in limbo in Congress as Democrats struggled to get all party members on board with higher spending on issues such as paid family leave and free preschool.
Wyden’s remarks reinforce the idea that any slimmer spending bill is likely to include many of the proposed health care and clean energy provisions. Sen. Joe Manchin (D-W.Va.), who has opposed other aspects of the package, has claimed he supports reforms to prescription drug pricing.
President Joe Biden has pushed for the legislation as one of his signature policy programs, and yesterday he called for the Senate to act. “We have to get this done,” he said in a message to supporters at an event hosted by health consumer group Families USA. Any new package will need Manchin’s vote, as Democrats have only the narrowest-possible majority in the Senate and have no Republican backing for another trillion-dollar spending bill.
That legislation included broad parts of Democrats’ health agenda. It would extend higher tax credits for people who buy health insurance via the Affordable Care Act’s individual marketplaces, direct the federal government to demand lower prices for some prescription drugs, and place a $35 per month cap on insulin costs for insured Americans. Wyden said there remains support for retaining these items, as well as extending coverage to residents in the dozen states that have declined to expand their Medicaid programs under Obamacare. Read more from Alex Ruoff.
Also on Lawmakers’ Radars
Senators Push Overhaul of Health Agencies: Two influential senators are calling for an overhaul of the country’s public health programs in a new bill that would change how the government monitors disease outbreaks, stockpiles supplies, and responds to future pandemic threats. The measure, released as a draft yesterday, also aims to refocus the mission of the Centers for Disease Control and Prevention. It’s being pushed by Senate Health, Education, Labor and Pensions Chairwoman Patty Murray (D-Wash.) and ranking member Richard Burr (R-N.C.).
In a push to bolster U.S. preparedness for pandemics, the senators are calling for more federal leadership and accountability. The draft measure, called the PREVENT Pandemics Act, seeks to create a task force whose members would be appointed by bipartisan congressional leadership and assess the country’s preparedness for pandemics, while recommending improvements to Congress and the White House. The legislation also looks to spur better coordination between U.S. health agencies.
It would require the Senate to confirm the CDC director, and would establish clear functions for that role. One such responsibility would be strengthening U.S. genomic sequencing capabilities so the nation can more quickly detect variants and how swiftly they spread. The CDC director would also improve the reporting and exchange of health data.
Meanwhile, the bill would require U.S. regulators to improve clinical trials through digital strategies, and use real-world evidence to inform decisionmaking. It would also require the FDA to publish a report on best-practices for communicating with companies developing medical products. Notably, the legislation would change the way the FDA inspects facilities, reviews products, revises labels, and penalizes the sellers of counterfeit devices. Read more from Riley Griffin.
- The pandemic preparedness bill broadly intends to avoid problems that popped up with Covid-19 and raises questions about the HHS agency tasked with overseeing the national stockpile and the unit’s overall ability to respond to a public health emergency. Read more from Jeannie Baumann.
Eshoo Joins Democrats in Calling for Lower Medicare Premiums: House Energy and Commerce Health Subcommittee Chair Anna Eshoo (D-Calif.) called on the Centers for Medicare and Medicaid Services to reduce monthly premium costs for Medicare Part B beneficiaries this year. Eshoo’s request comes as CMS plans a 14.5% hike in Part B premiums partly due to the high price of Aduhelm, Biogen’s controversial Alzheimer’s treatment that was first priced at $56,000 per year. It’s since cut the price by half, spurring calls from Democrats to roll back the premium hike. Read Eshoo’s letter here.
The Coronavirus Pandemic
Nursing Homes Call Staffing Report Mandate ‘Tone Deaf’: Nursing homes, struggling with record staff shortages and surging Covid-19 infections, will soon have to publicly report their employee turnover rates and weekend staffing levels for nurses, a move designed to help consumers select quality facilities. Much of the new information, set to post this month on Medicare.gov’s Care Compare website, will not be flattering. That’s because “every nursing home in the country, for the most part, is struggling” with high employee turnover and low staffing, said David Grabowski, a professor of health-care policy at Harvard Medical School.
The new details are expected to show what recent research has already found—that staffing levels for registered nurses, licensed practical nurses, and nurse aides usually drops off on weekends. And many nursing home employees are leaving their jobs within a year. Both problems are closely tied to poor-quality care. As nursing homes try to restore public confidence after Covid-19 killed at least 186,000 residents in long-term care, the reporting mandate could provide an untimely black eye for an industry already on the ropes. Read more from Tony Pugh.
- Meanwhile, nonprofit aging services providers want Biden and Congress to give 4.6 million nursing home workers one-time payments of $2,000 apiece—and a $5 an hour hike—in order to shore up nationwide staffing shortages at the facilities. The request was one of six sent to Biden by Katie Smith Sloan, CEO of LeadingAge, an industry group that represents more than 5,000 nonprofit aging services providers, including nursing homes. Read more from Tony Pugh.
- At the same time, hospitals want an antitrust investigation into price gouging by nurse staffing agencies, warning that they’re paying expensive prices to keep emergency rooms staffed to care for patients with Covid-19. The American Hospital Association urged the Biden administration to scrutinize what they call anti-competitive pricing by nurse staffing firms that charge hospitals costly rates for health-care providers amid unprecedented demand, and collectively raise their prices. Read more from Alex Ruoff.
- Also in Covid-19 aid, the U.S. will distribute more than $2 billion in Provider Relief Fund payments to over 7,600 medical providers this week to alleviate the impact of the Covid-19 pandemic, according to a statement from the Health and Human Services Department. These payments follow the nearly $9 billion in funding that was released by HHS in December of 2021. The funds cover such costs as personnel, recruitment and retention, and supplies. Read more here.
Shot-or-Test Rule Abandoned After Supreme Court Loss: The Biden administration is withdrawing its Covid-19 shot-or-test rule for workers at large employers following a Supreme Court ruling that blocked the measure from taking effect. The announcement was posted yesterday in the advanced version of the Federal Register. Federal lawyers followed that notice with a motion asking a Cincinnati-based federal appeals court to toss out the pending legal challenge to the emergency temporary standard, arguing the withdrawal of the rule will moot the case. Read more from Bruce Rolfsen and Robert Iafolla.
- Biden’s bid to boost vaccinations among workers has hit a red wall of Republican-appointed judges, particularly those jurists tapped by the Trump administration. Twelve of the 14 lower court rulings against Biden’s suite of workplace vaccine rules were handed down by either GOP-appointed district judges or GOP-controlled appellate court panels, according to a Bloomberg Law review of cases. Robert Iafolla, Erin Mulvaney, and Kathleen Dailey have more.
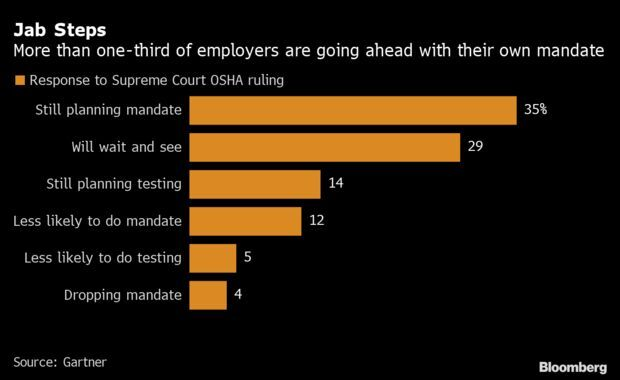
- Nevertheless, over a third of U.S. employers still plan to implement a vaccine mandate. Thirty-five percent of companies polled by Gartner last week said the court’s ruling on Jan. 13 won’t derail their plans to require vaccinations, compared with just 4% that said they’re now dropping their mandate. A further 29% haven’t made a decision yet, but 12% said they’re now less likely to impose a requirement. Read more from Matthew Boyle.
U.S. Still Playing From Behind With Inadequate Testing: The U.S. is awash in vaccine doses, but the availability of Covid-19 tests has been an issue throughout the most intense spikes of the pandemic. That’s because while there was an Operation Warp Speed to create vaccines, there hasn’t been a comparable initiative for tests. In response to omicron, the Biden White House has stepped up its investments in testing. There’s a risk that without longer-term investments in testing infrastructure, the U.S. will again find itself scrambling when the next highly contagious variant comes along. Cynthia Koons and Emma Court have more.
Omicron Surge Still Threatens Hospitals, CDC Says: Rates of Covid-19 severe illness and death fell in the U.S. amid the omicron surge compared with the delta wave as well as last winter’s wave, although high case counts and deaths continue to threaten patients and health systems, according to a government report. Still, even as the proportion of hospital beds used for patients’ treatment rose under omicron, ICU admissions for the same patients dipped slightly, the Centers for Disease Control and Prevention said in a report published yesterday. Read more from Angel Adegbesan.
- Meanwhile, a decision to test an omicron-specific booster is “prudent” in case the variant persists, Anthony Fauci said on MSNBC after Pfizer announced it’s starting a study of a Covid-19 vaccine specifically targeting the variant. But Fauci, the chief medical adviser in the Biden administration, added that the U.S. is seeing omicron peak and a variant specific booster may ultimately not be needed, Norah Mulinda reports.
- Related: Pfizer, BioNTech Begin Study of Omicron-Specific Covid-19 Vaccine
Students With Disabilities Get Mixed Mask Mandate Results: Parents of students with disabilities in Iowa and South Carolina yesterday received mixed results in suits saying state provisions barring mask mandates in public schools violate their children’s rights. The Eighth Circuit upheld—but narrowed—an injunction that barred state officials from enforcing a law blocking masking requirements in districts with students with disabilities. And the Fourth Circuit released South Carolina Gov. Henry McMaster (R) from a suit over a budget rule barring districts from using state money to support mask rules. Mary Anne Pazanowski has more.
More Headlines:
- Deaths Months After Covid-19 Cases Point to Pandemic’s Grim Aftermath
- WHO’s Tedros Nominated for Second Term, as Covid-19 Keeps Spreading
- China Criticizes U.S. Diplomats Seeking Exit Over Covid Rules
- China’s Pandemic Blame Game Fizzles Over ‘Infection-by-Mail’ Theory
- Covid-19 Paid Leave for California Workers Coming, Newsom Says
- Some Trial Judges Halt Cases Over Concerns With Unvaccinated Jurors
- DeSantis Wants U.S. to Allow FDA-Shunned Covid-19 Drugs
What Else to Know Today
Insurers Falling Down on Mental Health Parity, Agencies Say: Health plans and insurers are failing to deliver parity in mental health coverage as required by law, the Biden administration said. The Departments of Labor, Health and Human Services, and the Treasury yesterday issued their 2022 Report to Congress on the Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act of 2008 (MHPAEA). Of more than 1,000 parity analyses requested of health plans by DOL, “We unfortunately did not receive one that we viewed as sufficient,” DOL Acting Assistant Secretary for the Employee Benefits Security Administration Ali Khawar said in a press call. Read more from Sara Hansard.
Patient Voices Get Focus in Guidance for Device Studies: Medical device makers should use rating scales, questionnaires, and other tools to collect patient feedback on products’ safety and effectiveness, the Food and Drug Administration said in guidance aimed at better integrating patient voices in product studies. The FDA’s two final guidance documents released yesterday aim to tackle repeated complaints from patient groups that device makers fail to adequately consider their perspectives in clinical trials, which they say limits the effectiveness of the products marketed for them. Read more from Celine Castronuovo.
Court Upholds Right to Appeal Medicare Patient Reclassifications: Medicare beneficiaries admitted to a hospital as inpatients but later reclassified as outpatients are entitled to appeal the change, the Second Circuit has ruled. The lack of an appeals process denies patients the right to due process under the U.S. Constitution, the Second Circuit said, affirming a ruling from a lower court. Christopher Brown has more.
FDA Gets Lukewarm Response on Tobacco Efforts: The Food and Drug Administration got mixed reviews for its tobacco control efforts last year in American Lung Association’s 20th annual State of Tobacco Control, which was released today. The agency won praise for saying it would remove menthol cigarettes and other flavored tobacco goods from the U.S. market this year, as well as criticism for failing to remove many e-cigarette products from store shelves last year. The ALA also noted that states have come a long way over the past 20 years: there are now 28 “smokefree states” that ban indoor smoking. Read the report here.
More Headlines:
- California’s ‘Medicare for All’ Bid Hinges on Unconvinced Democrats
- CVS, Aurobindo Skirt Death Lawsuit Over Generic Blood Pressure Pill
- Walgreens Skirts Another Lawsuit on Babies’ Pain Reliever Deception
- Philip Morris Is Rejected Again in Efforts to Resume Iqos Sales in U.S.
With assistance from Alex Ruoff
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.