HEALTH CARE BRIEFING: Democrats Prepare Sweeping Pharma Hearings
By Alex Ruoff and Brandon Lee
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
The House Oversight and Reform Committee’s 20-month-long investigation into how drugmakers set their prices concludes this week, with two days of hearings featuring executives from six major pharmaceutical companies.
The committee has been collecting documents and interviewing pharmaceutical company personnel since it first launched the probe in January 2019, committee aides said. The investigation sought information on how companies spend their profits on research for new drugs versus advertising or executive pay, as well as how they determine when to hike the price of their products.
The hearings, set for Wednesday and Thursday, will give members the chance to showcase their findings and make the case that the government needs to take a direct hand in controlling the price of medicines in the U.S.
“These companies sell medications that are critical to our health and well-being, but their skyrocketing prices are simply unsustainable,” Rep. Carolyn Maloney (D-N.Y.), chairwoman of the Oversight Committee, said in a statement.
Lawmakers will hear from executives from Celgene, Bristol-Myers Squibb, Teva, Amgen, Mallinckrodt and Novartis. The prices of blockbuster drugs sold by these companies, such as the cancer medication Revlimid acquired by Bristol-Myers Squibb from Celgene, have steadily risen in recent years.
Leaving this Congress with just another scolding from lawmakers may be a win for the pharmaceutical industry. This year is set to end without the passage of any major drug pricing legislation, despite the cost of prescription medicine being a central campaign issue for both President Donald Trump and Democrats.
Trump has recently attempted to sidestep Congress on the issue, seeking to use his executive power to affect what Americans pay at the pharmacy, with dubious results. While House Democrats passed a bill that would direct the government to demand drugmakers lower the cost of some medicines, the administration and most Republicans opposed the measured out of concerns it could hurt U.S. drug companies.
In the Senate, Finance Chairman Chuck Grassley (R-Iowa) managed to navigate a bipartisan slate of drug pricing measures, including one to cap price increases, but his committee never gathered enough support for passage.
Groups that advocate for getting the federal government to more-aggressively limit drug prices say the coronavirus pandemic made it more difficult to pass a drug pricing bill, as drugmakers are now playing a key role in creating a vaccine to stop the spread of the virus. But they said the issue isn’t going away anytime soon and the table is set for action next year.
Alex Lawson, executive director of the group Social Security Works, a left-leaning advocacy group that backed House Democrats’ drug pricing legislation, said that “the chess pieces are still set up for something to happen.”
Happening on the Hill
Azar Testifies on Covid: Health and Human Services Secretary Alex Azar is set to testify on the pandemic before the House Select Subcommittee on the Coronavirus Crisis today.
Vaccine Safety: The House Energy and Commerce Subcommittee on Oversight and Investigations plans a hearing Wednesday on producing a safe and effective Covid-19 vaccine.
Bills on House Floor: The House is scheduled to consider the following bills this week under suspension of the rules, which requires a two-thirds majoirty for passage:
- Stem Cell Research Programs: The C.W. Bill Young Cell Transplantation Program and the National Cord Blood Inventory Program would be reauthorized for five years under H.R. 4764. For more, see the BGOV Bill Summary by Danielle Parnass.
- Anti-Doping Agency: The U.S. Anti-Doping Agency would be reauthorized through fiscal 2029 under H.R. 5373. For more, see the BGOV Bill Summary by Brittney Washington.
- Pediatric Drug Vouchers: The Food and Drug Administration’s priority review vouchers for rare pediatric disease treatments would be extended for four years under H.R. 4439. For more, see the BGOV Bill Summary by Danielle Parnass and Alex Ruoff.
- Breast Cancer Awareness Programs: Programs at the Centers for Disease Control and Prevention to raise public awareness of breast cancer and provide resources for early detection would be reauthorized under H.R. 4078. For more, see the BGOV Bill Summary by Brittney Washington.
- Medicaid Maternity Coverage: States could extend Medicaid coverage to women for one year after they give birth under H.R. 4996, which also would eliminate a cap on certain Medicaid drug rebates. For more, see the BGOV Bill Summary by Sarah Babbage.
- Allergy and Asthma Grants: The Health and Human Services Department would have to give preference for asthma-related grants to states that require schools to have allergies and asthma management programs under H.R. 2468. For more, see the BGOV Bill Summary by Danielle Parnass.
- South Asian Heart Health Grants: Grant programs to support heart-disease research and awareness among South Asian communities in the U.S. would be authorized by H.R. 3131. For more, see the BGOV Bill Summary by Brittney Washington.
- Family Support for Addiction: HHS would award grants to nonprofit groups that provide services for families of individuals struggling with addiction under H.R. 5572. For more, see the BGOV Bill Summary by Naoreen Chowdhury.
- School Health Centers: Funding for school-based health centers would be reauthorized through fiscal 2024 under H.R. 2075. For more, see the BGOV Bill Summary by Danielle Parnass.
- Tribal Health Data: Federal agencies would have to share public health data with American Indian tribes under H.R. 7948, which also would reauthorize the National Center for Health Statistics for five years. For more, see the BGOV Bill Summary by Danielle Parnass.
- Mental Health Disparities: Additional funding and programs to address racial and ethnic minority mental health disparities would be authorized by H.R. 5469, including an extra $650 million annually for five years for the National Institute on Minority Health and Health Disparities and $100 million annually for five years for the National Institutes of Health. For more, see the BGOV Bill Summary by Danielle Parnass.
- School Mental Health Services: Grants to support mental health services at schools for children experiencing trauma and violence would be authorized by H.R. 1109. The measure would authorize $130 million annually from fiscal 2021 through 2024 for the Health and Human Services Department to award the grants and contracts. For more, see the BGOV Bill Summary by Danielle Parnass.
- Emergency Mental Health Care: Hospitals could receive grants to support patients after they treated for a mental health episode at the emergency department under H.R. 2519. For more, see the BGOV Bill Summary by Danielle Parnass.
- Emergency Room Suicide Screening: HHS would have to establish a program to award grants to as many as 40 health-care facilities to improve their screening and treatment of emergency department patients who are at risk for suicide under H.R. 4861. For more, see the BGOV Bill Summary by Naoreen Chowdhury.
- Student Suicide Awareness: State, local, and tribal education agencies would have to implement school policies for student suicide awareness and prevention training to receive certain federal mental health funding under H.R. 7293. For more, see the BGOV Bill Summary by Naoreen Chowdhury.
- Behavioral Intervention Guidelines: HHS would have to develop best practices to help schools establish behavioral intervention teams under H.R. 3539. For more, see the BGOV Bill Summary by Naoreen Chowdhury.
- Covid-19 Resurgency Plans: Federal agencies would have to develop plans to address a resurgence in Covid-19 cases under H.R. 7496. For more, see the BGOV Bill Summary by Michael Smallberg.
- Pregnant Inmate Health: The Bureau of Prisons would be required by H.R. 7718 to ensure that appropriate pregnancy and childbirth services and programs are provided to women in its custody. The bill also would prohibit restrictive practices on pregnant and postpartum women, and it would authorize a grant program to support provision of incarcerated women’s health care. For more, see the BGOV Bill Summary by Adam M. Taylor.
The Coronavirus Pandemic
Early Treatments Could Be ‘Bridge’ to Vaccine: Monoclonal antibodies that stop Covid-19 from spreading in the body are among promising strategies for averting severe illness from the virus before vaccines arrive, according to the country’s top infectious diseases director. Antibody-based medications, other blood products from recovered patients, and antivirals are being investigated for their potential as early treatments, Anthony Fauci said, Jason Gale reports.
FDA Demanded to Keep Away Politics: Prominent scientists took the unusual step of demanding the FDA commissioner publicly guarantee political pressure won’t interfere with the approval process for a Covid vaccine. Their open letter, which was co-siged by two former top FDA scientists, calls on Commissioner Stephen Hahn to reaffirm that science—and not politics—will steer the FDA’s regulatory decisions. Read more from Jeannie Baumann.
Novavax Kicks Off 10,000 Patient Vaccine Study: Novavax announced plans to start enrolling participants for a late-stage study of its experimental shot for the coronavirus in 10,000 patients in the U.K. The drugmaker joins the ranks of AstraZeneca, Pfizer, BioNTech and Moderna as its vaccine candidate enters the final stretch on the path toward regulatory approval. There are about 38 shots being tested in humans around the world and over 140 others in earlier stages of trials, World Health Organization estimates show. Cristin Flanagan has more.
More Headlines:
- China Sees Vaccine Capacity to Hit 1 Billion Doses by End-2021
- Nursing Home Managers Get Criminal Charges in Nation’s First
- New York State Virus Cases Top 1,000 for First Time Since June
- Virginia Governor Northam, Wife, Test Positive for Coronavirus
What Else to Know
Democrats Focus on Health Care in Opposing Trump Court Pick: Democrats zeroed in on the risk to Americans’ health-care coverage in previewing the tactics they’ll use to oppose the confirmation of Amy Coney Barrett, Trump’s new Supreme Court pick. Regardless, Barrett’s confirmation seems assured given the Republican majority in the Senate. She would replace the most progressive member of the Supreme Court, Justice Ruth Bader Ginsburg, who died on Sept. 18. Barrett’s a conservative favorite who, at just 48 years old, could be expected to remain on the court for decades.
Democrats indicated a laser-like focus on the Affordable Care Act, or Obamacare, and portrayed the election as a way for their voters to help offset the court’s expected further shift to the right under Trump. “The antidote to his — whatever he does is to vote, vote, vote,” Speaker Nancy Pelosi (D-Calif.) said on CNN’s “State of the Union” yesterday. “Vote for affordable care, vote for your preexisting condition, vote for your safety, and vote for your health.”
The Supreme Court will hear a case Nov. 10 that could ultimately be the means to strike down the ACA, long a Republican goal. Read more from Michael Riley and Tony Czuczka.
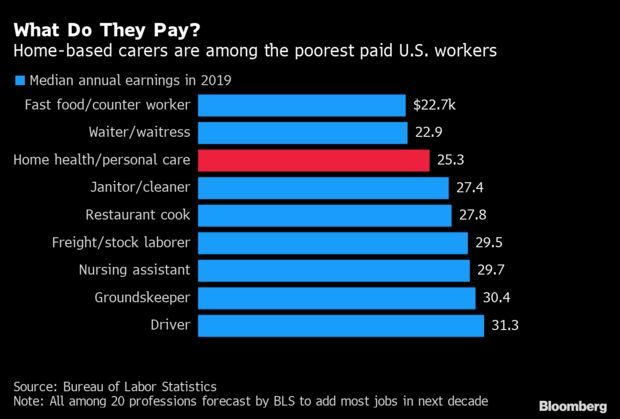
Race, Gender Disparities in Home-Health Care: Home-based health care, the nation’s fastest-growing industry and a key employer for Black women, remains one of America’s poorest paying sectors. Demand for home-care has surged as the population ages, but rapid hiring in the industry went into reverse over the past six months, with Black and female unemployment skyrocketing above the overall rate. Roughly 110,000 home-care jobs vanished in March and April, and less than half have since returned. Read more from Katia Dmitrieva.
More Headlines:
- California Law Resolves Federal Conflict For Health Care Data
- California Governor Vetoes Genetic Testing Data Disclosure Law
- Bausch, Eton Get FDA Approval for Preservative-Free Eye Drops
- Akebia’s Lawsuit Over Medicare Pay for Kidney Drug Proceeds
- Vertex Says FDA Approved Kalydeco for Cystic Fibrosis in Infants
To contact the reporters on this story: Alex Ruoff in Washington at aruoff@bgov.com; Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.