HEALTH CARE BRIEFING: Burr Pushes CDC-Private Sector Cooperation
By Brandon Lee
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
A sweeping pandemic preparedness bill aims to encourage CDC collaboration with the private sector, an effort to break down an agency culture one senior Republican described as so insular that it contributed to testing delays and other problems early in the pandemic.
“I think it’s cultural because there’s a fight to produce anything that’s not internally generated,” Sen. Richard Burr (R-N.C.), the ranking member of the Senate Health, Education, Labor and Pensions Committee as well as the architect of the 2006 Pandemic and All Hazards Preparedness Act and its two reauthorizations, said during a briefing yesterday.
Burr and HELP Chair Patty Murray (D-Wash.) released last week a draft version of their latest pandemic bill, known as the PREVENT Pandemics Act, which calls for several changes to the Centers for Disease Control and Prevention. It would also authorize the CDC’s director to continue activities related to developing capabilities for disease outbreak forecasts and analysis, “including by leveraging the capabilities of public and private entities,” according to a bill summary.
Historically the CDC “has no public-private partnership or relationship,” Burr said. But Burr cited collaborations with the private sector in Operation Warp Speed as key in developing and distributing the Covid-19 vaccines in record time. “The bill is heavy on CDC reform,” he said. “Because to successfully achieve the type of framework we need for the future, it’s going to require three things: leadership, communication, and innovation.” Read more from Jeannie Baumann.
Happening on the Hill
Spotlight on Biogen Alzheimer’s Drug in User Fee Hearing: Biogen’s Alzheimer’s treatment was a main focus for lawmakers at the year’s first hearing on renewing the user fee agreements that will help fund the FDA. Members of the House Energy and Commerce Committee’s health panel expressed confusion over Medicare’s preliminary decision to cap coverage of Aduhelm to patients enrolled in trials. “I was shocked to find out that the CMS proposed national coverage determination severely restricts Medicare for a whole class of Alzheimer’s treatments,” Rep. Cathy McMorris Rodgers (R-Wash.) said.
McMorris Rodgers, the top Republican of the full committee, and others pointed to a disconnect between the decisions made by the FDA and the Centers for Medicare & Medicaid Services. Patrizia Cavazzoni, head of the FDA’s Center for Drug Evaluation and Research, responded that the two agencies make decisions “independent of each other,” and that the Medicare agency made its own decision “on the basis of their own parameters and standards.” Read more from Celine Castronuovo.
- Biogen’s shares slid 5% after Aduhelm had just $1 million in sales in the fourth quarter. Aduhelm has faced a series of setbacks since gaining approval from the FDA in June. The clearance was granted in spite of opposition from the agency’s outside scientific advisers, who said that clinical trial data didn’t clearly show that the drug worked as intended. And since then, insurers have balked at paying for it. Read more from Robert Langreth and Angelica Peebles.
- Meanwhile, Eli Lilly is delaying its application for approval of its experimental treatment for Alzheimer’s, after U.S. health officials restricted reimbursement for the class of drugs. The request for accelerated approval now won’t be complete until after the first quarter, company executives said yesterday. Lilly said it would complete the filing in 2022, without specifying exactly when. The Indiana-based drugmaker had said that it could see approval for the therapy, donanemab, in the second half of 2022. Read more from Riley Griffin and Robert Langreth.
Long Covid-19 Disability Revives Push to End Medicare Wait: Congress must make it easier for people who are disabled by long-term Covid-19 conditions to access Medicare benefits, advocates and doctors told a House Ways and Means Committee hearing yesterday. Anyone eligible for Social Security Disability Insurance is also eligible for Medicare, but people who get approved for SSDI have to wait a mandated 24 months before they can get those health benefits. That wait can be harmful to those suffering from a chronic illness, such as long Covid-19, one doctor said. Read more from Lydia Wheeler.
The Coronavirus Pandemic
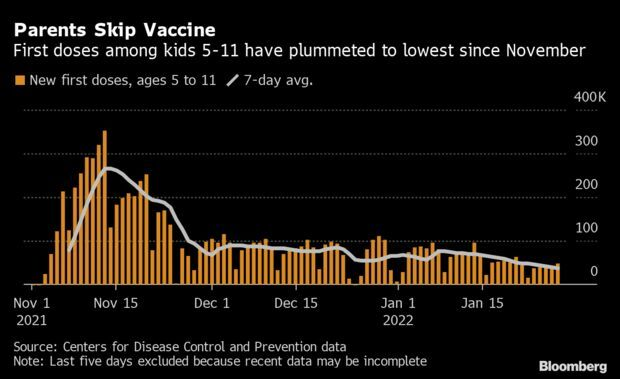
Kids’ Vaccinations Fall as U.S. Considers Shots for Youngest: Vaccinations among children ages 5-11 have fallen to the lowest levels since the shots were first approved, a sign that parental enthusiasm for the shots may be running low even as authorities consider expanding the shots to even younger kids. The seven-day average of first doses fell to about 37,062 on Jan. 28, marking the slowest one-week period since the U.S. approved the vaccines for those children on Nov. 2, data from the CDC show. Just 31% of kids 5-11 have gotten a shot, compared with 75% of the total population. Read more from Jonathan Levin.
Medicare Will Cover Home Covid-19 Tests: Medicare beneficiaries will be able to get up to eight free over-the-counter Covid-19 tests per month beginning in early spring, the Biden administration announced. The new initiative will allow Medicare to directly pay participating pharmacies and other entities for over-the-counter tests approved or authorized by the FDA. Private Medicare Advantage plans may offer coverage of the tests as a supplemental benefit in addition to hospital Part A coverage and outpatient Part B benefits, the Centers for Medicare & Medicaid Services said. Read more from Tony Pugh.
Merck, Lilly Treatment Sales Top Estimates: Merck and Eli Lilly both saw stronger-than-expected sales of their Covid-19 drugs in the fourth quarter—the period when the highly contagious omicron variant first arrived in the U.S. Merck’s Covid-19 antiviral molnupiravir brought in $952 million, surpassing estimates of $842 million, according to the firm’s earnings statement yesterday. The treatment appears to fend off omicron well, and along with Pfizer’s similar oral antiviral pill, is likely to be more widely used in the months ahead as supplies ramp up. Read more from Timothy Annett and Riley Griffin.
Vaccine Rule Lawsuit Against Kentucky Hospital Ends: A private Kentucky hospital shed a challenge over its Covid-19 vaccine mandate after health-care workers asked to dismiss the case based on a recent Supreme Court decision. The U.S. District Court for the Eastern District of Kentucky granted the plaintiffs’ request with prejudice, meaning they cannot refile it in the future. Read more from Mary Anne Pazanowski.
More Headlines:
- Covid-19’s Endemic Shift Means Slowdown for Virus Product Makers
- Europe Could Enjoy Covid-19 ‘Ceasefire’ With Immunity Boost: WHO
- WHO Says BA.2 Sub-Variant of Omicron Has Spread Across Africa
- Novavax Protein-Based Vaccine Gets British Drug Regulator’s OK
- Pardes Biosciences Gets FDA Clearance of IND for Covid Antiviral
- California Covid-19 Paid Leave Bill Clears Key Senate Committee
What Else to Know Today
FDA Targets Licensed Drug Distributors: Wholesale drug distributors would have to establish written policies to ensure their facilities are fully capable of storing and transporting prescription drugs to qualify for licensure under a rule proposal from the FDA. The proposal would establish national standards for licensing to ensure supply chain participants are properly vetted and qualified to distribute drug products. “These measures are intended to help protect American consumers from drugs that may be counterfeit, stolen, contaminated, or otherwise harmful,” the FDA said. Read more from Allie Reed.
More Headlines:
- Pfizer’s Hospira Wins Legal Fees in Ruling by Biden Circuit Pick
- Swisher’s ‘Legal Limbo’ on Cigars Fails to Get Order Versus FDA
- Practicefirst Wins Bid to Toss Out Health-Care Data Breach Case
- NFL Player Should Get More Than Eight Years for Health Care Fraud, DOJ Says
- Astra Sues Alembic, Natco to Block Copies of Calquence Drug
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.