HEALTH CARE BRIEFING: Biden to Host Vaccine Summit, Pledge Doses
By Brandon Lee
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President Joe Biden will call for 70% of the world to be vaccinated by this time next year during a virtual vaccine summit he’ll host today that’s intended to spur countries, businesses and organizations to set firm targets to defeat the coronavirus pandemic.
Biden will pledge a U.S. order of 500 million doses of Pfizer-BioNTech’s vaccine for donation abroad, pushing the total U.S. donation pledge above 1.1 billion doses as he leans on other nations to do the same, according to officials familiar with the event.
The summit attendees will include foreign leaders, private sector figures and representatives of non-governmental organizations, and include a mix of speeches and recorded statements. Biden will lead one of four sessions, on vaccinating the world, while Vice President Kamala Harris will lead another.
The Pfizer doses will be produced in the U.S. and be shipped through Covax, the global vaccine sharing system, to low and lower-middle- income countries, beginning in January and running through next September.
The officials, speaking on condition of anonymity before the summit took place, declined to say how much the order would cost, but said the contracting process is continuing. The doses will be provided on a not-for-profit basis, they added. The company plans to produce them at four plants across the U.S. Pfizer confirmed the deal in a statement.
The pledge is on top of a 500-million-dose donation announced in June at the Group of Seven summit in the U.K. Distribution of those vaccines began last month. Combined with shipments made so far of surplus supply, the U.S. donation total is now at least 1.13 billion doses, more than double the total delivered domestically. Read more from Josh Wingrove.
More on the Pandemic
FDA Weighing Boosters After CDC Panel Vote: The Food and Drug Administration is expected to decide as soon as today on a recommendation for Covid-19 booster shots made by Pfizer and BioNTech, two sources familiar with the matter said, the latest step in a process that could open the door to extra shots in the coming days. The agency’s decision would tee up consideration by an advisory panel of the Centers for Disease Control and Prevention, which has scheduled a meeting for later today and tomorrow to discuss boosters.
An FDA advisory panel on Friday rejected a call for boosters for all adults, instead opting to urge them for a narrower group: people 65 and older, people at risk of severe Covid-19, and people at risk of occupational exposure. The FDA could tweak those parameters, Josh Wingrove and Robert Langreth report.
Booster Dose of J&J Shot Has 94% Efficacy: Also in vaccines, a booster dose of Johnson & Johnson’s shot provided 100% protection against severe illness when given two months after the first dose, according to widely anticipated data that suggests it increases the potency of the one-time shot. The booster was 94% effective at preventing symptomatic Covid-19 infections in the U.S. portion of the Phase III trial and 75% effective overall when it was given 56 days after the initial dose, the company said. Robert Langreth has more.
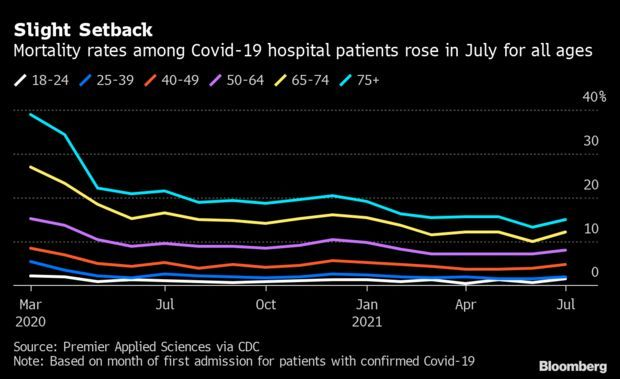
Fatality Rates Grow in U.S. Hospitals: Mortality rates among adult Covid-19 patients in U.S. hospitals rose in July from all-time lows, a slight setback in what has generally been a trend of steadily improving outcomes. Among cohorts 18 and up, patients hospitalized with Covid-19 in July were slightly more likely to die than they were in June, according to Premier Applied Sciences data. About 15% of patients 75 and older died, up from 13.2% in June. Those 65-74 died 12.3% of the time, up from 10%. Read more from Jonathan Levin.
U.S. to Pay $1.2 Billion for Rapid Tests: The federal government is spending nearly $1.2 billion to purchase 187 million rapid Covid-19 tests from Abbott Laboratories and Celltrion, according to a Health and Human Services official. The deals, which include options to buy more, are part of an investment of about $2 billion announced by the White House earlier this month to make the rapid tests more available. The U.S. is also leveraging the Defense Production Act to build out capacity. Shira Stein and Emma Court have more.
OSHA Exposed to Culture Wars Over Vaccine: The federal government’s workplace safety arm has proven resilient to controversy during its half-century existence, but Biden‘s vaccination directive has propelled it into American culture wars, putting its reputation as a by-the-book agency on the line. After surviving decades of corporate lobbying and efforts to defang it, the Occupational Safety and Health Administration must now confront the toxic disputes surrounding Covid-19 public health mandates. Ben Penn has more.
More Headlines:
- U.S. Small Businesses Rethink Reopenings Amid Covid-19 Risk
- Retailers Seek 90 Days to Comply With OSHA Vaccination Rule
Happening on the Hill
Hearings Today:
- Nomination: The Senate Finance Committee meets for a hearing on the nomination of Christi A. Grimm to be inspector general for the Department of Health and Human Services.
- Virus & Children: The House Energy and Commerce Subcommittee on Oversight and Investigations plans a hearing on Covid-19’s effect on children.
- Virus Relief Programs: The House Select Subcommittee on the Coronavirus Crisis meets for a hearing on pandemic relief programs.
Stopgap Punts Fentanyl Analogue Ban to 2022: Restrictions on fentanyl-like substances would extend into early next year under a stopgap spending measure the House passed yesterday, temporarily delaying a debate on the overdose crisis. The stopgap measure (H.R. 5305) would push the expiration of the government’s power to ban the powerful drugs to Jan. 28, 2022. Those powers are currently set to expire Oct. 22. Congress has repeatedly extended the authority temporarily, going back to the Trump administration.
The White House earlier this month asked to permanently place all fentanyl-analogues on the list of the most controlled narcotics to give law enforcement agencies the power to prosecute anyone caught in possession of them illegally. But some lawmakers, namely Sen. Cory Booker (D-N.J.), wanted these authorities to expire earlier this year, arguing that they contribute to over-policing of people struggling with addiction. Read more from Alex Ruoff.
Poll Shows Support for Home Care in Biden Plan: A survey released yesterday by the U.S.’s largest union of home health care workers showed 81% of Americans who responded supported the home health care provisions proposed in Biden’s sweeping economic agenda that Congress is now considering. The poll was conducted by the Service Employees International Union, which will hold a rally at the U.S. Capitol tomorrow along with groups such as the American Society on Aging and Caring Across Generations.
What Else to Know Today
U.S. Urges Supreme Court to Uphold Roe v. Wade: The federal government urged the U.S. Supreme Court to reaffirm the right to an abortion and strike down Mississippi’s ban on abortions after 15 weeks. If “states are permitted to ban pre-viability abortion, the effects are likely to be felt most acutely by young women, women of color, and those of lesser means,” the administration said in its brief. Decades of reliance on Roe v. Wade and Planned Parenthood v. Casey requires the court not jettison those decisions, it added.
“Indeed, all women now of childbearing age (and presumably most of their partners) have grown up against the backdrop of Roe and Casey’s core holding,” the government argues. Earlier this month, the Supreme Court allowed a Texas abortion ban to go into effect, saying procedural issues kept the justices from stepping in. The Texas law bans abortions after six weeks and like the ban in Mississippi doesn’t include an exception for rape or incest. Oral arguments begin Dec. 1. Kimberly Strawbridge Robinson has more.
- Meanwhile, the first two lawsuits filed in the wake of a Texas bill that allows private citizens to sue doctors who perform abortions in all but the earliest weeks of pregnancy may have trouble gaining traction. That’s because the lawsuits against Alan Braid, a San Antonio doctor who wrote a newspaper column in which he admitted to violating rules that forbid abortions after about the sixth week of pregnancy, were filed by people who believe the procedure should be legal. And lawsuits generally must have two sides in disagreement in order to get to trial, making the plaintiffs’ beliefs relevant here. Read more from Laurel Brubaker Calkins and Lydia Wheeler.
- Related: Ban or Regulation: Missouri Abortion Law Hinges on Contrast
FDA, Drugmakers Agree on Biosimilar User Fees Deal: Drugmakers could having an easier time getting cheaper, interchangeable biological products across the finish line under a new FDA effort to facilitate their development as part of the next deal on user fees. The Food and Drug Administration released a letter outlining its commitments to industry for the next round of biosimilar user fees for fiscal 2023 through fiscal 2027. User fees paid by drug companies help fund FDA operations. Read more from Jeannie Baumann.
Employers on Hook for Mental Health Parity: Health insurers are now in the crosshairs of the Department of Labor’s aggressive enforcement of mental health parity, but it’s unlikely to mean employers will escape scrutiny. In what appeared to be a first for the department, DOL initiated litigation last month against an insurer to ensure health plans offering mental health and substance use disorder benefits are covering treatments at the same level as physical care. Lydia Wheeler and Sara Hansard have more.
More Headlines:
- Aetna Barred From Suing Over North Carolina Medicaid Bid Denial
- Sesen Bio Hit With Shareholder Suit Over Cancer Drug Disclosures
- J&J Sees Baby Powder Asbestos Claims Revived in California Case
With assistance from Shira Stein
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.