HEALTH CARE BRIEFING: Abbott, FDA Agree to Restart Formula Plant
By Brandon Lee
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Abbott Laboratories reached a pact with federal authorities that would allow it to begin making baby formula again at a troubled plant in Michigan, a move that could help ease a supply shortfall that has rattled many parents.
Under the terms of the agreement, which was filed on Monday by the Justice Department in a federal court in Michigan, Abbott must take steps prescribed by the Food and Drug Administration to assure it can safely produce formula. The agreement is still subject to the approval of the court, the FDA said Monday. Abbott must meet the FDA’s food standards, among other measures, when it decides to resume production, the agency said.
That plant has been shut since February after four children who had been fed formula made there were sickened by cronobacter bacteria, and two died. No further cases of children being sickened by formula have since been reported, the Centers for Disease Control and Prevention said. Abbott said that its products are not to blame for the illnesses and deaths.
The shutdown helped make an already-existing shortage of formula far worse, and the crisis has intensified in recent weeks, with stores seeing inventories exhausted by panic buying. State and federal law-enforcement officials say they are also on the lookout for price gouging. In addition to the agreement with Abbott, the FDA laid out standards for importing formula made by overseas companies on Monday. Read more from Anna Edney.
- The shortage is pushing more parents to explore an even pricier alternative: Human breast milk supplied by donor banks. Inquiries for donor milk have risen by 20% since the start of the shortage, said Lindsay Groff, the director of the Human Milk Banking Association of North America. That follows a record year of distribution: HMBANA’s member banks doled 9.2 million ounces out to families and hospitals of babies in need in 2021, a 22% increase from 2020, Groff said. Ella Ceron has more.
- Read more: Addressing the Baby Formula Shortage: FDA Powers Explained
Also on Lawmakers’ Radars
Tuesday’s Hearings:
- The Senate Appropriations Labor-HHS-Education Subcommittee holds a Tuesday hearing on the National Institutes of Health’s fiscal 2023 budget request. Acting NIH Director Lawrence Tabak and National Institute of Allergy and Infectious Diseases Director Anthony Fauci are among those scheduled to testify.
- The House Science, Space and Technology Committee scheduled a markup Tuesday for legislation including H.R. 7180, which authorize the National Science Foundation to award grants to support research on the disruption of regular cognitive processes associated with Covid-19.
- The House Appropriations Military Construction-Veterans Affairs Subcommittee holds a Tuesday hearing on electronic health record modernization at the Veterans Affairs Department.
- The House Oversight Coronavirus Crisis Subcommittee holds a Tuesday hearing on the Covid pandemic’s effects on the nation’s workforce of low-wage women.
- BGOV Calendar: See the full list of Tuesday’s hearings.
Bills Set for Passage: The House plans to vote Tuesday on measures related to military veterans’ health care under suspension of the rules—which requires a two-thirds majority—including:
- H.R. 7500, which would authorize major medical facility projects for the Veterans Affairs Department. The bill would authorize $3.4 billion in fiscal 2022 to restore or construct 12 VA medical facilities. The House Veterans’ Affairs Committee hasn’t considered the bill. For more, see the BGOV Bill Summary by Christina Banoub.
- H.R. 5754, which would require the VA’s Office of Patient Advocacy would have to create an online system for veterans to file and track complaints about VA health-care services. The House Veterans’ Affairs Committee approved the bill by voice vote on April 6. For more, see the BGOV Bill Summary by Christina Banoub.
CBO Reviews Deficit Impact of Lower Medicare Age: Lowering the age to join Medicare to 60 would increase federal deficits by $155 billion between 2026 and 2031 and bring 7.3 million new people in the public program, according to an analysis by the CBO. The Congressional Budget Office analysis outlined the effect of allowing more people to join Medicare on Medicaid and employer plans, showing 150,000 people would retire early since they’d no longer need a job for coverage, Alex Ruoff reports. Read it here.
The Coronavirus Pandemic
US Extends Public Health Emergency Again: The US government will extend the Covid-19 public-health emergency past mid-July, continuing pandemic-era policies as the nearly 2 1/2-year outbreak drags on. The Health and Human Services Department has repeatedly renewed the public-health emergency since implementing it in January 2020. The declaration allows the federal government to grant emergency authorizations of drugs and vaccines, and enabled millions to get coverage through Medicaid.
National health organizations, including the American Hospital Association, the American Medical Association and the American Academy of Pediatrics, have lobbied for the public health emergency to be extended. Last week, they wrote to Secretary Xavier Becerra urging the Biden administration to maintain the emergency “until it is clear that the global pandemic has receded and the capabilities authorized by the PHE are no longer necessary.”
The Biden administration is extending the declaration as it simultaneously calls on lawmakers to approve billions in additional funding for pandemic response. Meanwhile, Republicans have encouraged the White House to unwind the declaration, which was enacted via the Public Health Service Act. When the emergency period ends, the US will lose certain powers and likely roll back many programs that pharmaceutical companies, telehealth providers and insurers have relied on. Riley Griffin has more.
Vaccine Protection Better Than Records Show: Widely used methods for counting US Covid hospitalizations can make vaccines appear less effective than they actually are, according to a group of Boston-based researchers. The researchers argue their work raises questions about how the US ought to approach future booster-shot campaigns and how the country measures the severity of Covid. It may also have implications for bonus payments for hospitals caring for Covid patients. Drew Armstrong has more.
China Spat May Spoil Deal for Vaccine I.P. Waiver: A brewing trade fight between the US and China may unravel a nearly two-year effort to ease intellectual-property rules for making Covid vaccines and cast more doubt on the World Trade Organization’s reputation as a negotiating forum. Biden’s top trade official in Geneva said any WTO agreement related to Covid vaccines must explicitly exclude China from being able to benefit from the deal. Read more from Bryce Baschuk.
More Headlines:
- Business, Hospital Groups Join Call for Rehearing in Covid Suits
- Is It Covid or the Flu? New At-Home Test Spots Multiple Viruses
- North Korea Deploys Military to Fight 1.5 Million ‘Fever Cases’
What Else to Know Today
Conflicting Laws in Northwest Expose Post-Roe Chaos: The stark divisions and murky legalities of how states handle abortion rights may be most intense at the northwestern part of the US. Less than two months before a leaked Supreme Court document signaled Roe v. Wade’s imminent demise, the governors of Washington and Idaho signed diametrically opposite bills on abortion. Now, Washington’s clinics are preparing for a flood of Idahoans seeking care. Read more from Dina Bass and Mat Day.
- George P. Bush, who’s eyeing the GOP nomination for Texas attorney general, said he would support further efforts to limit abortion if he’s elected, including rules punishing companies that pay for employees to travel to other states for such procedures. Bush said lawmakers should ban businesses that provide such benefit from doing business with the state. He also pledged to crack down on mailed abortion pills to Texans. Shelly Hagan has more.
FDA to Be ‘Deeply Involved’ in ARPA-H: The FDA’s ability to advance biomedical discoveries from Biden‘s new research hub will hinge on an efficient partnership and will likely intensify an ongoing debate over accelerated approvals, the agency’s second-in-command said. Janet Woodcock’s comments on Monday offered some of the first glimpses into how the FDA sees its role as the Advanced Research Projects Agency for Health (ARPA-H), gets off the ground. Read more from Jeannie Baumann.
Employers Pay Hospitals Over Double Medicare Prices: Employers and health insurers paid hospitals more than double what Medicare would have paid in 2020, according to a RAND report Tuesday. Prices paid to hospitals during 2020 by employers and private insurers for inpatient and outpatient services averaged 224% of what Medicare would have paid, RAND said in its fourth report comparing private insurance payments to health providers to Medicare rates. Sara Hansard has more.
LGBTQ Couple’s IVF Hopes Hinge on Fertility Definition: The Biden administration is considering a requirement that some health coverage plans cover fertility treatment for policyholders regardless of their sexual orientation or gender identity. The White House could use a pending Obamacare update to tackle the definition of infertility often used by insurers to determine eligibility for in-vitro fertilization, which is impossible for some LGBTQ people to meet. Shira Stein has more.
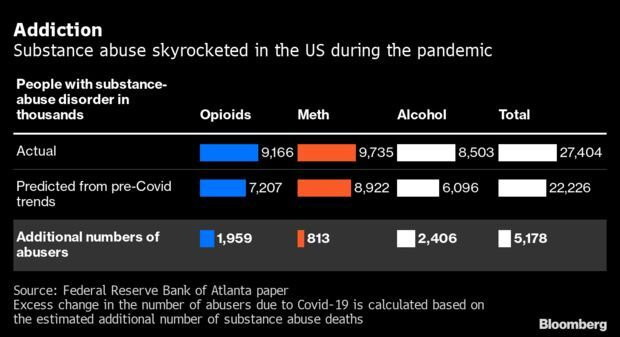
Drug-Abuse Spike Hampered Labor Recovery: A rise in drug abuse during the Covid-19 pandemic has hampered the recovery in the American labor market, according to a recent paper published by the Federal Reserve Bank of Atlanta. Elevated substance abuse accounted for 9% to 26% of the decline in the workforce participation of workers age 25 to 54—the so-called prime-age—between February 2020 and June 2021, said the authors of the study. Read more from Alex Tanzi.
More Headlines:
- High Court Wants US’s View on Whistleblower Medicare Fraud Claim
- 3M Turned Down by US Supreme Court on Medical Blanket Lawsuits
With assistance from Alex Ruoff
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.