HEALTH CARE BRIEFING: Biden Budget Set to Omit Major Health Vows
By Brandon Lee
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President Joe Biden is set to unveil a budget that would increase federal spending to $6 trillion in the coming fiscal year, with annual deficits of more than $1.3 trillion over the next decade, according to documents cited by The New York Times.
The proposal, set to be publicly unveiled today, offers a full accounting of Biden’s previously released plans for trillions of dollars in new taxes and spending while also revealing for the first time how the administration sees inflation and growth being affected by enacting his agenda.
Like most presidential budgets, the documents will be largely aspirational. Senate and House Democrats hold only narrow majorities, and lawmakers of both parties have made clear they’re unlikely to adopt Biden’s proposals in full.
The budget release builds on a $1.52 trillion discretionary spending request—that is, excluding required spending like Social Security—released by the White House in April that saw the president call for a 15.9% increase in domestic outlays, led by significant new investments in education and health-care programs.
But the biggest controversy may stem from what Biden omits from his budget. The budget only accounts for legislation the president is pursuing this year, leaving out key campaign promises like the enactment of an Affordable Care Act public option, lowering the Medicare eligibility age, or letting Medicare negotiate drug prices.
That’s spurred the ire of some progressives, who urged Biden to prioritize health-care issues early on in his administration. Read more from Justin Sink.
- Yesterday, more than 155 moderate and progressive House Democrats called on Biden in a letter to expand and improve Medicare as part of the American Families Plan, according to a statement. The Democrats, including Reps. Pramila Jayapal (Wash.) and Jared Golden (Maine), asked Biden to lower the Medicare age and allow it to negotiate drug prices. Read the letter here.
The Coronavirus Pandemic
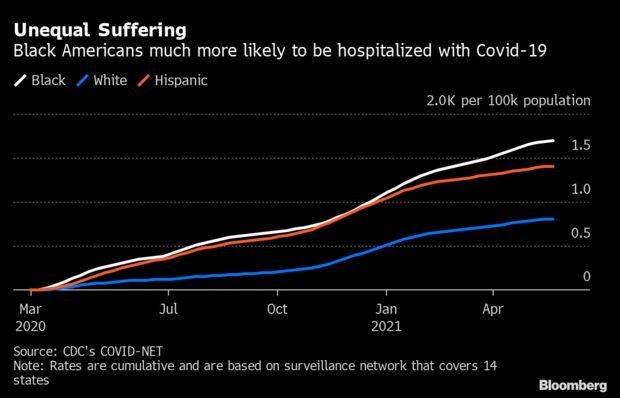
Inequities Persist Even as U.S. Pandemic Wanes: The Covid-19 pandemic in the U.S. is likely to end the way it started—with Black and Latino residents suffering a disproportionate amount of the pain and suffering. Though things have improved for all Americans, Black people are still being hospitalized with Covid-19 at double the rates of White people, according to Covid-Net, a hospital surveillance network for the Centers for Disease Control and Prevention.
The disparities reflect the same long-running inequities in health care and wealth that have contributed to higher rates of obesity and other medical conditions. But they also underscore the urgency for the U.S. to improve its vaccination campaign in minority communities. It’s clear Black and Hispanic communities want vaccines more than Republicans and White Evangelical Christians, and yet they’ve received fewer by comparison. Read more from Jonathan Levin.
Tech CEOs Pressed on Vaccine Myths: Facebook, Twitter and Google are facing new questions from House Democrats about myths spreading on their platforms about the coronavirus vaccines. The leaders of the House Energy and Commerce Committee sent letters to the CEOs of the three corporations after recent reports said misinformation about the pandemic continues to circulate online, Catherine Larkin reports. Read the letter here.
OSHA Pick Defends Covid-19 Rule: The question of whether a federal regulation is needed to protect workers from Covid-19 infection was the focus of the Senate confirmation hearing for Biden’s pick to lead OSHA, Doug Parker, who argued an emergency rule can withstand a legal challenge. Parker leads California’s Division of Occupational Safety and Health, where he oversaw a Covid-19 emergency rule enacted in November. Read more from Ben Penn and Bruce Rolfsen.
Royal Cruise Tests Federal v. State Rules: Royal Caribbean Cruises is cleared to resume cruises in the U.S. starting June 26 from Florida’s Port Everglades. The company is requiring everyone over 16 to present proof of vaccination, according to a statement, following one of two recommendations from the Centers for Disease Control and Prevention. But Florida banned so-called vaccine passports, and Republican Gov. Ron DeSantis (R) has insisted that the rule applies to cruise companies operating out of the state’s ports. It’s unclear how the cruise can sail from the Sunshine State as long as both rules stand. Read more from Bloomberg News.
More Headlines:
- Eli Lilly Receives DOJ Subpoena on Covid-19 Antibody Facility
- California Doling Out $116.5 Million in Prizes for Vaccinations
- New York Requires Paid Leave for Covid-19 Vaccine Side Effects
- China’s Sinopharm Unveils Awaited Details on Vaccine Study
- Seychelles Virus Mysteries Pit Anti-Vaxxers Against Scientists
What Else to Know Today
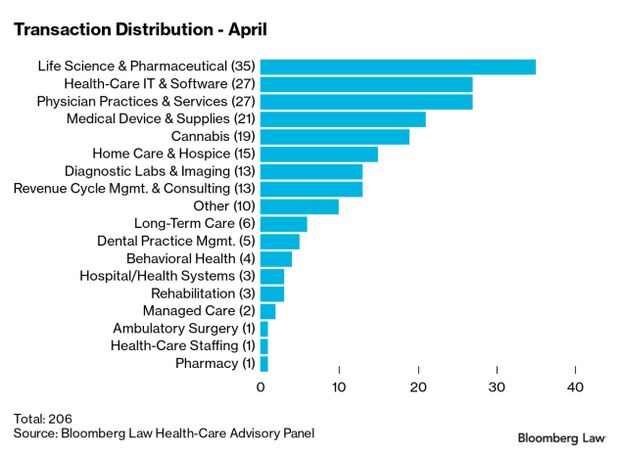
Health Deals Keep Pace as Pandemic Wanes: Health mergers and acquisitions continued at a hot pace in April with 207 deals either closed or announced. That’s the third month in 2021 that broke the 200-deal threshold. Only four months last year had up to 200 deals as the Covid-19 pandemic persisted, according to Larry Kocot, leader of KPMG’s Center for Healthcare Regulatory Insight. Projections of continued strong economic growth suggest that M&A activity will “remain robust for the coming months,” he said.
But the tax and health-care cost offset provisions in legislative packages favored by Democrats and Biden could create headwinds for the health-care and life sciences sector. Chances for passage of major bills in the narrowly divided Congress remain unclear, but incremental health reforms—such as possible drug-pricing legislation—appear possible as the parties start to position themselves for the 2022 elections, Kocot said. Read more from Christopher Brown.
More Headlines:
- HHS Partners With Investment Fund for Public Health Readiness
- Eli Lilly Gets Extra Time to Respond to Discount Penalty Threat
- Bristol Myers Gets FDA OK for Zeposia for Ulcerative Colitis
- Biohaven’s NURTEC ODT Approved by FDA for Migraine Prevention
- Provention Bio’s Drug to Delay Diabetes Gets FDA Panel Support
Editor’s Note: Bloomberg Government’s Health Care Briefing will not publish on Monday due to the Memorial Day holiday. We will return on Tuesday.
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.