HEALTH CARE BRIEFING: US Ranks 32 in Securing Covid-19 Vaccines
By Brandon Lee
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Thirty-one countries around the globe have reserved more Covid-19 vaccine per capita than the U.S., a Bloomberg analysis of vaccine agreements across the world shows.
Operation Warp Speed program is credited with shaving years off the typical development timeline for vaccines, which are now on the brink of being deployed. But after leading that effort, the U.S. has yet to exercise some options to lock down additional supplies that could offer further insurance against manufacturing or scientific problems.
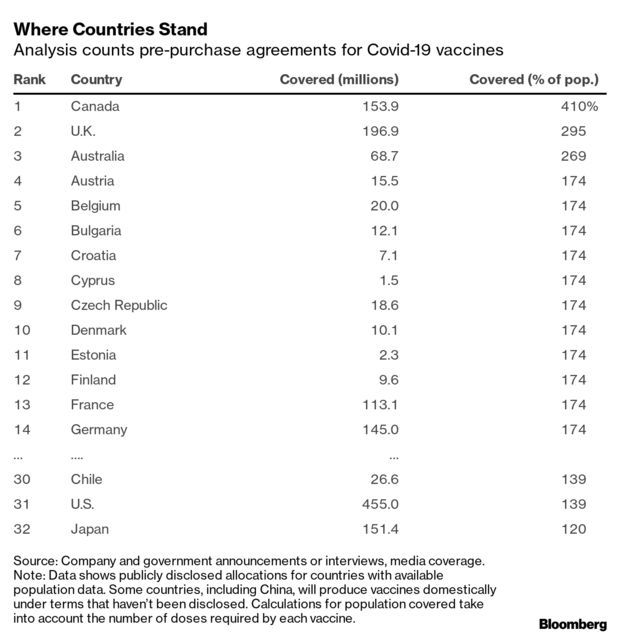
Bloomberg reviewed over 80 agreements between vaccine makers and countries around the globe to reserve allocations while they are still in development. Canada, the U.K. and Australia top the list, with enough vaccine doses reserved to cover their populations several times over.
The U.S. ranks 32nd in per-capita vaccine reservations, behind the 27 European Union nations that banded together to pre-order doses in larger quantities. The U.S. is sandwiched between Chile and Japan in 31st and 33rd, respectively, according to Bloomberg’s analysis.
“It’s a shocking abdication of government responsibility,” said Craig Garthwaite, the director of Northwestern University’s healthcare program at the Kellogg School of Management. “I’m so demoralized this will delay by another month or two getting the economy going.”
Those worries aren’t abstract. Pfizer, which makes the first vaccine expected to be authorized for use in the U.S., earlier this year reduced its near-term production targets. Another leading shot from AstraZeneca is expected to require further study in the U.S. to confirm its efficacy—delaying access to the inoculation.
The U.S. struck deals with Pfizer and Moderna, the two vaccine makers closest to getting their shots approved by federal regulators. But while the U.S. locked up the deal with Pfizer for 100 million doses of vaccine for its population of about 330 million, the EU pre-bought 200 million doses for its 450 million people. The EU also outpaced the U.S. in acquisitions from Johnson & Johnson and a vaccine being developed by Sanofi and GlaxoSmithKline. Read more from Drew Armstrong and Tom Randall.
- Less Than Half of Pfizer Shots Expected Will Ship At First: Meanwhile, less than half of the available 6.4 million doses of Pfizer’s shot will be initially sent out to states, and 500,000 will be held separately in reserve by the U.S. government, according to a senior official at Operation Warp Speed. Gustave Perna, the army general who serves as Warp Speed’s chief operating officer, said yesterday that the U.S. plans to distribute 2.9 million doses in the first tranche of shipments. Read more from John Tozzi and Angelica LaVito.
U.S. officials have sharply disagreed with the idea there will be any delay for most Americans to get the shots by the middle of next year and have stated they’re in talks to expand supply under the agreements. The U.S. is in negotiations with Pfizer and Moderna about expanding the country’s access to shots, Health and Human Services Secretary Alex Azar said yesterday. Read more from John Tozzi.
More on the Pandemic
Single Pfizer Shot Staves Off Covid-19: A single dose of Pfizer’s Covid-19 vaccine appears to be effective more than half the time, but doctors advised against skipping the second vaccine dose because of uncertainty over how long that will provide immunity. An FDA advisory panel will meet later today to discuss whether the agency should grant an emergency authorization for the Pfizer vaccine, which is effective roughly 95% of the time with two doses. But the new FDA analysis shows just one dose appears to be 52.4% effective. Read more from Jacquie Lee.
U.S. in Talks With Merck for New Treatment: Operation Warp Speed is in negotiations with Merck to secure supply of a treatment for the deadliest cases of Covid-19. As hospitalizations reach record highs in the U.S., Warp Speed chief scientific officer Moncef Slaoui said the talks with Merck for the under-the-radar drug, CD24Fc, ensued after the drugmaker acquired a 10-person biotechnology firm that spent decades making the intravenous treatment. Read more.
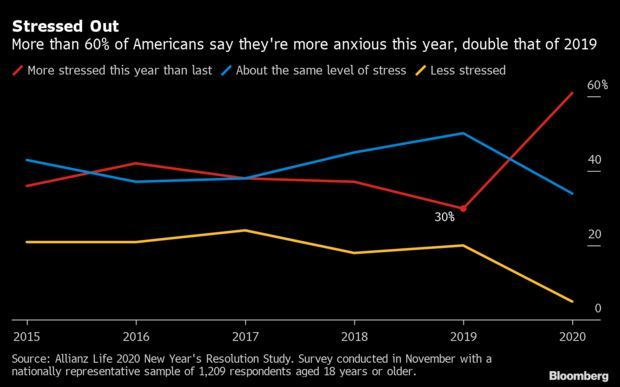
61% of Americans Feel More Stressed This Year: The coronavirus pandemic has left its mark on Americans’ mental wellbeing, according to Allianz Life’s 2020 New Year’s Resolution Study. Roughly 61% of respondents in the annual survey said they feel more stressed this year, more than double 2019’s level; 57% said health and wellness is their top focus area for the new year, up six percentage points and coming at the expense of financial stability, the main priority for less than a quarter of participants. Read more from Sophie Caronello.
The Future of U.S. National Stockpile Isn’t a Bigger Stockpile: The Strategic National Stockpile doesn’t have enough supplies to meet the federal government’s own targets. But in the long run, health officials don’t want to make it bigger. Their goal before the next Covid-size disaster is to make stockpiling itself less central. Read more from Shira Stein.
There’s Still Time to Beat Covid Without Lockdowns: South Korea’s successful approach of regimented masking, aggressive testing, and high-tech contact tracing is a blueprint for the U.S. and other democracies. Read more from Matthew Campbell and Heesu Lee.
More on the Pandemic:
- Pfizer Shot Gets Canadian OK; Vaccinations to Start Next Week
- U.K. Says Those With Severe Allergy Should Skip Pfizer Vaccine
- Pfizer Says Some Covid-19 Documents Accessed in Cyberattack
- Sinopharm’s Shot Has 86% Efficacy Against Covid-19, UAE Says
- England’s Schools Had Low Virus Risk After Reopening: Report
Happening on the Hill
Covid-19 Relief Deal Delayed With Pelosi, McConnell Holding Back: Senate Majority Leader Mitch McConnell (R-Ky.) and House Speaker Nancy Pelosi (D-Calif.) have given no sign yet that they’re ready to directly engage in negotiations to sort through competing pandemic relief proposals — a step that many lawmakers say will be necessary to complete a deal this month.
The Senate GOP leader is now on board with a $916 billion proposal released Tuesday by Treasury Secretary Steven Mnuchin, while the House speaker sees a rival $908 billion plan still being drafted by a bipartisan group of lawmakers as the best path to a deal to aid the struggling U.S. economy. Their positioning shows consensus emerging on an overall price tag. But the proposals differ on key features. Although their proxies are engaged in negotiations, the top congressional leaders haven’t yet planned a tete-a-tete to resolve the outstanding issues. Read more from Laura Litvan and Erik Wasson.
Doctors Build Bipartisan Support to Block Medicare Cut: Nearly 330 lawmakers are moving to stop new Medicare physician payment cuts from taking effect on New Year’s Day, with bipartisan support building to include such a provision in the year-end spending package. House and Senate members from both parties have either co-sponsored legislation or written to their leadership or HHS, seeking to stop the Medicare payment cuts in the 2021 Medicare physician fee schedule, according to the Surgical Care Coalition. The planned rate reductions are due to budget neutrality provisions in the Medicare Act that require program payment hikes be offset by equal reductions elsewhere. Read more from Tony Pugh.
Senators Call for ‘Fair’ Vaccine Distribution: Sen. Mark Warner (D-Va.) and others called on HHS Secretary Azar and CDC Director Robert Redfield in a letter “to ensure a fair and equitable vaccine distribution” as the FDA weighs whether to give emergency authorization to the Pfizer-BioNTech coronavirus vaccine. The letter follows Azar’s comment that states should decide who gets the first round of the vaccine, which Warner says “could lead to a patchwork of varying distribution plans and affect vaccine access for minority and high-risk populations.” Read the letter here.
Senate Passes Food Allergy Bill: The Senate passed an amended version of a bill addressing food allergies (S. 3451) from Sen. Tim Scott (R-S.C.). The legislation would add sesame to a list of major food allergens that need to be clearly identified on labels, beginning in 2023. It would also require the Health and Human Services Department to report and provide recommendations on federal food allergy regulation. The House passed a similar bill (H.R. 2117) by voice vote Nov. 17. Among other differences, that bill would authorize the FDA to designate major allergens by regulation, Sarah Babbage reports.
Electronic Health Data Standards: The House is set to consider under expedited procedure today a bill (H.R. 7898) that would clarify the Department of Health and Human Services inspector general’s authority to investigate technology developers and health-care providers that impede access to electronic health information. It would require HHS to consider whether health-care entities have recognized cybersecurity practices in place when it determines fines and audits related to data standards. Read the BGOV Bill Summary by Michael Smallberg.
Today’s Hearings:
- National Opioid Crisis: The Senate Homeland Security and Governmental Affairs Permanent Subcommittee on Investigations scheduled a hearing on oversight of legislation implemented to combat the national opioid crisis.
- Vaccine Transportation: The Senate Commerce, Science and Transportation Subcommittee on Transportation and Safety meets to examine the logistics of transporting a Covid-19 vaccine.
What Else to Know Today
Fall Regulatory Plan Debuts Before Biden’s Inauguration: A long list of regulations federal agencies had planned to advance or cut over the next year was released yesterday by the White House Office of Management and Budget, even though agency priorities will change as President-elect Joe Biden is sworn in on Jan. 20. The fall agenda included a number of new proposals or revised timelines for completion of rules. Among them:
- The Health and Human Services Department is planning to propose a rule in January to “robustly address” unlawful discrimination on the basis of disability.
- The Centers for Disease Control and Prevention is planning to issue an interim final rule in May 2021 to set new performance standards for fit tests for face masks.
Despite the coronavirus pandemic, federal agencies appear to be on track to issue a number of President Donald Trump’s high-priority regulations by the end of 2020. Among them are two rules intended to cut the price of prescription drugs Trump personally announced Nov. 20. Read more from Cheryl Bolen.
Drug Middlemen Shift Arguments to Escape Liability: Courts have been allowing powerful pharmacy drug middlemen to wield federal protections for employee benefit plans as both a sword and a shield. Pharmacy benefit managers, which administer drug benefit programs in employee health plans, have won cases in recent months by using the Employee Retirement Income Security Act to strike state regulations and escape liability for plan losses. PBMs have been enjoying the law’s benefits without paying its costs, lawyers say. Lydia Wheeler reports.
Most Americans Want Forceful ActionsAgainst Drug Costs: A majority of Americans want the federal government to take forceful steps to lower prices of prescription drugs, including manufacturing generics, according to a poll given to Bloomberg Government by a progressive polling group. The Data for Progress poll showed that policy proposals, including allowing the government to negotiate with drugmakers, remains popular with Democrats and Republicans alike. Nearly 70% of Democrats and 65% of Republicans said they back drug cost negotiation, Alex Ruoff reports. Read the full report here.
More Headlines:
- Eli Lilly’s CEO David Ricks Becomes Chairman of PhRMA Board
- Generic Maker Asks SCOTUS to Review Gilenya’s Patent Ruling
- Lantheus Wins FDA Fast Review for PyLTM for Prostate Cancer
- Minerva Neurosciences’ Schizophrenia Drug Stirs Class-Action
With assistance from Alex Ruoff
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.