HEALTH CARE BRIEFING: Biden Says Trump Antagonizing Virus Effort
By Brandon Lee and Alex Ruoff
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
President-elect Joe Biden yesterday assailed the Trump administration’s refusal to cooperate on the presidential transition, which he said hinders his team’s ability to get up-to-date information on the coronavirus pandemic.
Biden said he couldn’t “fathom” President Donald Trump’s refusal to concede the election, saying he’ll “go down in history as being one of the most irresponsible presidents.”
Biden urged the Trump administration to let his team obtain details on a coronavirus vaccine and plans for distribution. “I would like to know exactly what this administration has in mind in terms of their Operation Warp Speed and how they plan it,” he said.
Biden said he hasn’t ruled out legal action to force the General Services Administration, which has so far declined to sign an ascertainment that Biden won the election, to begin the process for the transition. Until such paperwork is signed, Biden cannot get national security briefings, access to government officials, or real-time data on the coronavirus.
Biden also spoke with state governors who briefed him on the status of the pandemic in their states as the virus surges around the country. “Governors made clear that beating Covid-19 will require all of us working together,” Biden said. Noting that state and local budgets have been devastated by pandemic-related slowdowns, he said, “We’ve got to come together. The federal government has to deliver this relief.”
“My transition team hasn’t been able to get access to information we need” on virus testing, vaccine development and distribution, Biden said to the executive committee of the National Governors Association, made up of five Republican and five Democratic governors, in a video conference. Read more from Justin Sink, Erik Wasson, and Tyler Pager.
- At the same event, Biden added yesterday that his administration would not impose a national shutdown in response to the coronavirus pandemic. “I am not going to shut down the economy, period,” he said. He said the executive committee also discussed mandates for mask-wearing. It “shouldn’t be a partisan issue,” Biden said. “It’s not a political statement. It’s a patriotic duty.” Read more from Justin Sink.
Meanwhile, the White House coronavirus task force yesterday held its first press briefing since April, where coordinator Deborah Birx urged Americans to be vigilant as the pandemic surges nationwide. “Every American needs to be vigilant in this moment because we know when you are, we can stop this spread together,” she said. She also recommended Americans restrict in-person gatherings to the people they live with, with Thanksgiving holiday a week away. Mario Parker, Jordan Fabian, and Josh Wingrove have more.
Progressives Pressure Biden on Health Agenda: More than a dozen progressive groups are pushing Biden to stock federal agencies with people who favor their domestic policy agenda. The Progressive Change Institute, the Center for Popular Democracy, MoveOn, Public Citizen, the Sunrise Movement, the Economic Policy Institute and other left-wing groups sent a list of 400 names to the Biden transition team recently.
The list includes Adam Gaffney, the president of Physicians for a National Health Program, a group that advocates for a single-payer health system in the U.S., Andrew Kolodny, medical director of opioid policy research at Brandeis University and Lori Kearns, legislative director for Sen. Bernie Sanders (I-Vt.) and the author of Sanders’s “Medicare-for-All” bill.
“Getting these people into government would be a real victory,” Caitlin Lang, a spokeswoman for the Progressive Change Campaign Committee, part of PCI, said. Lang said the list is meant to build Biden’s hires throughout government, not just high profile posts that require Senate confirmation. Hiring off the list would send signals to progressive groups that the Biden White House is working toward their policy goals, she said.
WHO Advises Doctors Not to Use Remdesivir
The World Health Organization recommended against using Gilead Sciences’s remdesivir to treat hospitalized Covid-19 patients less than a month after U.S. regulators granted the drug a speedy approval.
“There is currently no evidence that it improves survival or the need for ventilation,” a panel of WHO-convened experts developing Covid-19 treatment guidelines said in The BMJ medical journal.
The recommendation is a blow to Gilead’s drug, which was one of the first thought to offer a meaningful benefit in treatment of coronavirus patients after a study showed it reduced their recovery time. The antiviral has been used widely to treat Covid and was among the drugs Trump received when he was diagnosed with the disease in early October.
The experts made the recommendation after the results of a global trial sponsored by the WHO, called Solidarity, found last month that remdesivir didn’t reduce deaths. They also reviewed data from three other trials and said the drug “has no meaningful effect” on the time it took patients to clinically improve. Read more from Anna Edney.
Happening on the Hill
Rift in Pandemic Insurance Eyed at Hearing: House lawmakers aired differences over the scope of proposals for covering insurance claims during future pandemics, part of an ongoing legislative discussion to undergird struggling small businesses.
A House Financial Services subcommittee hearing yesterday offered a venue for lawmakers and insurers to pitch ideas about how best to structure—or find alternatives to— one such program, outlined in a bill introduced by House Oversight and Reform Chairwoman Carolyn Maloney (D-N.Y.). They also debated whether insurance is the right avenue for rescuing businesses from the next pandemic. Read more from Jacob Rund.
Bezos Asked for Data on Covid-19 Rates for Workers: Five lawmakers are asking Amazon CEO Jeff Bezos for data on Covid-19 infection rates among Amazon’s workers. The group, including Sens. Elizabeth Warren (D-Mass.) and Sanders, called on the billionaire in a letter to release the virus data. Amazon released a report in October saying more than 19,000 employees have tested positive for coronavirus, but the lawmakers said the report was incomplete, Rebecca Kern reports.
GAO Praised for Study on Gun Violence Health Costs: Sen. Warren, a member of the Senate Health, Education, Labor, and Pensions Committee, Rep. Maloney, and Rep. Robin Kelly (D-Ill.) in a letter yesterday praised the Government Accountability Office’s announcement it would analyze the country’s health-care costs associated with gun violence, a request they had made in February this year. The study “is a good step towards confronting and addressing this crisis,” the lawmakers said. Read the GAO’s statement here.
Leadership Staff Met on Omnibus, Discussed Virus Aid: Staff from the offices of Speaker Nancy Pelosi (D-Calif.), Senate Majority Leader Mitch McConnell (R-Ky.), Senate Democratic Leader Chuck Schumer (D-N.Y.) and House Republican Leader Kevin McCarthy (R-Calif.) met yesterday to discuss an omnibus spending package. The talks on the spending plan are on a separate track from stalled negotiations on a Covid-19 relief bill, but during the meeting, the staff members discussed assistance measures tied to coronavirus a senior Democratic aide said without giving details. A House Republican aide said that expiring programs related to the pandemic such as extended unemployment insurance and the Paycheck Protection Program were on the agenda, along with other of expiring government programs, Erik Wasson and Billy House report.
The Coronavirus Pandemic
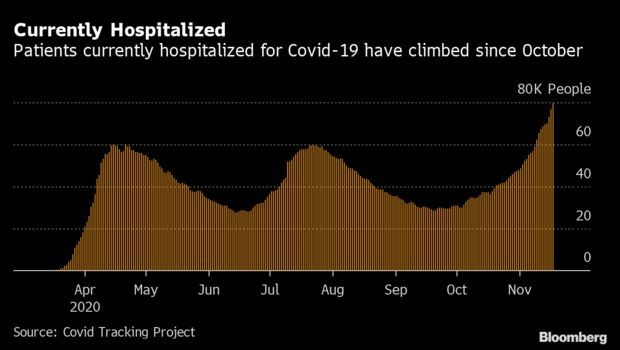
Covid-19 Hospitalizations Near 80,000: American hospitals are reeling as another spike in cases threatens to overwhelm capacity and drive up deaths. Almost 80,000 patients are hospitalized with Covid-19 in the U.S., another high in a week that’s pushed up the record every day since Nov. 10. California, Texas, and Illinois have made up nearly a quarter of all hospital stays. The Midwest saw immense pressure on its health system, while North and South Dakota hospitals saw the highest number of inpatients on Wednesday when scaled for population, roughly one out of every 1,685 residents, according to Covid Tracking Project data.
The uptick comes despite improving treatments and falling mortality rates from the disease. Doctors have greatly expanded the array of treatments available to tackle Covid-19. However, some must be administered in hospitals, potentially prolonging a patient’s stay. Read more from Nic Querolo and Timothy Annett.
Washington DC Region Sets Case Record: Coronavirus infections continue to rise in the greater Washington region, with more than 5,000 new cases reported yesterday between DC, Maryland and Virginia, a daily record. Daily cases in the region have averaged 4,109 over the past week and doubled since late October. Read more from Bloomberg News.
Covid Vaccines in FDA Express Lane Still Have Hurdles to Clear: Pfizer and BioNTech are on the brink of requesting emergency authorization of their Covid vaccine, and it could take at least three weeks for a U.S. Food and Drug Administration decision as trial data is probed by agency staff and outside advisers. Shown to be 95% effective and without any major safety issues, their vaccine could be the first to be cleared for use, but first it must undergo a thorough vetting.
A key step along the way is a meeting of outside FDA advisers, all experts in infectious diseases and vaccines. They’re set to confer Dec. 8-10, according to a person with knowledge of the situation. The FDA will spend the few short weeks between the emergency authorization request and the meeting sorting through the trial data. Read more from Anna Edney.
Study Confirms Astra Shot’s Response in Elderly: The University of Oxford confirmed that the Covid-19 vaccine it’s developing with AstraZeneca produced strong immune responses in elderly adults in an early study, with key findings from the last phase of tests expected in the coming weeks. The results, published yesterday in The Lancet medical journal, shed light on preliminary data published in recent months showing that the experimental shot generated an immune response in the elderly, who are at highest risk of severe illness. Read more from James Paton and Suzi Ring.
- Meanwhile, the University of Oxford’s head trial investigator said people should be able to mix Covid-19 vaccines so they would not be precluded from trying a different vaccine if their first is less effective. “The theory is that that should work, and there’s no reason” why it wouldn’t, Andrew Pollard, who’s leading the Oxford’s Covid-19 vaccine trial with AstraZeneca, said at a press briefing. Suzi Ring has more.
CDC Urges Against Traveling for Thanksgiving: With Thanksgiving only a week away, the Centers for Disease Control and Prevention urged Americans to skip holiday travel this year. The recommendation released by the CDC was a break from earlier messaging in which U.S. officials have largely declined to issue firm guidance for holiday gatherings, instead leaving those decisions to American families. The agency suggests virtual Thanksgiving gatherings, outdoor gatherings, and smaller or shorter gatherings as less risky alternatives. Read more from Kristen V. Brown and Josh Wingrove.
More Headlines:
- FDA Issues EUA for Lilly’s Combination for Treatment of Covid-19
- EU Says BioNTech, Moderna Shot Approval Possible in December
- ‘Keep Grandma Safe,’ Colleges Implore Students Heading Home
- Indoor Dining Darkens Across U.S., Deepening Restaurants’ Pain
- Traders’ Vaccine-Stock Hope Fades Amid Doubts on Road Ahead
- There’s More Lysol Than Ever, Yet It’s Still Not Enough in the U.S.
- Facebook Tagged 167 Million Posts for Virus Misinformation
- Data Heroes at Covid Tracking Project Fill U.S. Government Void
What Else to Know
Drug Rule Targeting Pharma Liaisons Clears Review: Release of a monumental drug policy that would scrap legal protections for certain payments between drugmakers and middlemen is imminent after it left executive review. The White House Office of Management and Budget completed action on the rule yesterday, according to its website, which is a quick turnaround after it received the revived regulation from the Health and Human Services Department Nov. 13. Read more from Jacquie Lee.
- Separately, rules to lift restrictions on physician financial arrangements and coordinated patient care cleared final White House review and could be published soon. The Office of Management and Budget completed action on the rules on Wednesday, according to its website. The long-awaited changes to two major laws that fight fraud and corruption in the health-care sector are aimed at streamlining the process for doctors to treat patients holistically rather than on a traditional fee-for-service basis. Fawn Johnson has more.
More Headlines:
- Conservatives Decry Trump’s ‘Most Favored Nation’ Drug Order
- Medical Lab Lawsuit Over HHS Repayment Thrown Out of Court
- CBD’s Differing Effects on Women and Men Under FDA Scrutiny
- Medicare Office Eyes A.I. to Help Find Errors in Medicare Claims
- Evolus Decision in Botox Battle Postponed by U.S. Trade Agency
- EPA Plans to Act on Medical Sterilizing Gas’ Cancer Risks
- Millions of Full-Time Workers Rely on Federal Health Care and Food Programs (GAO)
To contact the reporters on this story: Brandon Lee in Washington at blee@bgov.com; Alex Ruoff in Washington at aruoff@bgov.com
To contact the editors responsible for this story: Zachary Sherwood at zsherwood@bgov.com; Giuseppe Macri at gmacri@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.