HEALTH CARE BRIEFING: Senate Panel Examines Growth of Telehealth
By Brandon Lee and Alex Ruoff
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
The Senate Health, Education, Labor, and Pensions Committee is set to examine telemedicine, which lets patients meet with health-care providers electronically, at a hearing today as the sector sees substantial growth during the Covid-19 crisis.
Convinced that telehealth, which exploded as stay-at-home orders were issued, is here to stay, insurers are aiming to expand their networks and provide virtual primary health-care plans at lower premiums and less cost-sharing for patients.
When the novel coronavirus first struck, many insurers waived out-of-pocket costs so consumers could get care without exposing themselves or their health-care providers to the risk of coronavirus infection. The federal government and many states issued rules or enacted emergency laws to boost reimbursements for providers and ease restrictions, such as licensing requirements, to expand access.
Patients via telemedicine can receive health services at home from providers outside their regions, including monitoring for such chronic conditions at diabetes. More insurers, spurred by the lower costs and greater efficiencies, are setting up networks outside their geographic areas, potentially boosting competition among providers.
But telehealth has its limits, and it’s also unclear if the savings long associated with virtual medicine will be undermined by the higher rates that insurers and the federal government has paid to help expand the industry in the first place. Read more from Sara Hansard.
Congressional Virus Efforts
Clyburn Probing Nursing Home Deaths: House Majority Whip James Clyburn (D-S.C.), chairman of the House’s coronavirus panel, sent letters to the five largest U.S. for-profit nursing home companies as part of an investigation of Covid-19 deaths. He also sent a letter to the Centers for Medicare and Medicaid Services, the office that oversees nursing homes, according to a statement from the panel. Recipients include: Genesis HealthCare, Life Care Centers of America, Ensign Group, SavaSeniorCare and Consulate Health Care. Alex Ruoff has more.
FDA Streamlining Seen as Long-Term: One long-lasting effect of the Covid-19 pandemic could be that the FDA continues to green light some laboratory-developed tests, Rep. Michael Burgess (R-Texas) said yesterday. Burgess, speaking at a virtual event hosted by the conservative Heritage Foundation, said shifting responsibility for authorizing some lab tests to the FDA from Centers for Medicare & Medicaid Services has proven fast and effective during the pandemic and could be made permanent.
“I don’t think we need to go back to where we were before,” said Burgess, who is the top Republican on the House Energy and Committee Committee’s health subcommittee, Alex Ruoff reports.
Bill Targets Liability Waivers for Rallies: Rep. Mark Pocan (D-Wis.) unveiled legislation yesterday that would “prevent liability waivers from being enforced for indoor gatherings of 1,000 or more people” if the 14-day Covid-19 case trend is increasing in the locality, according to a statement. The measure aims to prevent President Donald Trump‘s campaign from being free of liability, should supporters at the president’s rallies get infected by the coronavirus. Read the bill’s text here.
Union Head Warns of Government Reopening Risk: The American Federation of Government Employees National President Everett Kelley told a House Homeland Security panel yesterday reopening federal agencies too early without proper protocols risked worsening outbreaks among employees, according to a prepared statement.
Health Care Inequality: The House Energy and Commerce Health Subcommittee scheduled a hearing today on racial and ethnic disparities in Covid-19 care and the health care system. Committee Chairman Frank Pallone (D-N.J.) yesterday wrote to Centers for Medicare & Medicaid Services Administrator Seema Verma to reiterate his request the agency release demographic claims data for the virus based on race, ethnicity and gender.
Senate Judiciary Hearing: The Senate Judiciary Committee will hold a hearing on June 23 to assess China’s culpability over the coronavirus pandemic.
Testing, Treatment & Research
Cheap Drug Saves Patients’ Lives in U.K. Study: A low-cost, widely used anti-inflammatory drug helped survival in patients with Covid-19, the first treatment to show life-saving promise months into the pandemic. Deaths among patients who needed breathing assistance were lower over a period of four weeks when they took the 60-year-old drug dexamethasone, Oxford University researchers said yesterday. The study was halted early because of its critical findings. Read more from John Lauerman.
Vaccine May Be Free for Vulnerable: Covid-19 vaccines will be provided free for anyone who cannot afford the vaccine, a senior administration official told reporters at a briefing on Operation Warp Speed. The Trump administration is also in talks with commercial health insurers, which have expressed interest in waiving co-pays, the official said. The program is the White House’s partnership campaign with drugmakers to develop a Covid-19 vaccine. The U.S. is hoping to distribute 300 million doses by January, Jeannie Baumann reports.
NIH to Continue Hydroxychloroquine Trials: The National Institutes of Health will continue with clinical trials on the potential for hydroxychloroquine to treat those hospitalized with Covid-19 despite federal regulators revoking the drug’s emergency authorization, the NIH said in an email. The NIH trial, titled “Orchid,” began enrolling patients in April and is screening those who may be susceptible to erratic heart rhythms, who are at risk when taking HCQ. Read more.
Masks Prevented Virus’ Spread, Study Says: Mandating the use of face masks among the public prevented up to 450,000 cases of Covid-19 in the U.S., a study published in Health Affairs yesterday concluded. Researchers viewed state and local requirements for public use of face masks between April and late May and found they reduced the spread of the coronavirus. Find the study here.
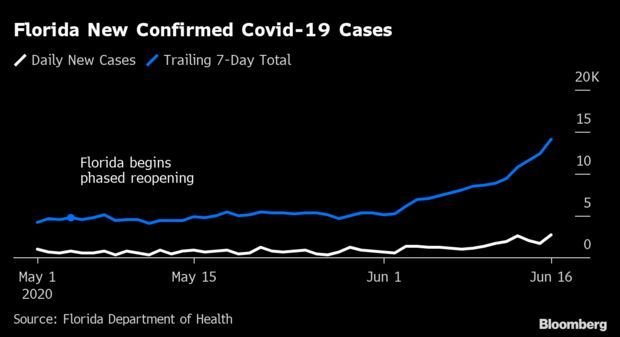
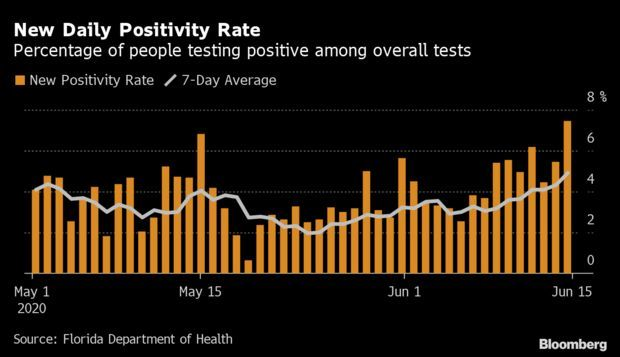
Florida’s Coronavirus Surge:Florida’s new Covid-19 cases surged yesterday to the highest level ever, extending a concerning upswing in confirmed infections six weeks after Gov. Ron DeSantis (R) began a phased reopening of the state.
Cases rose by 2,783, or 3.6%, to 80,109, compared with an average of 2.5% in the previous seven days. Read more from Jonathan Levin.
Summer Heat Waves Further Threaten Those Most at Risk From Covid: Despite Trump’s hopes, Covid-19 has shown few signs of abating with the warmer weather, and has in fact spiked in a number of states. Now, many health and city planners predict the crisis could get much worse as the U.S. faces what is expected to be one of the hottest summers on record.
The poor, the elderly, and people of color could face the toughest times yet as they are disproportionately being impacted by the coronavirus, and the economic recession will make it harder for many from these communities to afford basic utilities. The situation will be particularly dangerous for those stuck at home with Covid-19 symptoms, who don’t have—or can’t afford to run—air conditioning. It’s much worse, of course, for those who won’t have a place to stay at all, as temporary eviction suspensions end. Read more from Maya Earls.
More Headlines:
- HHS Taking on More Oversight of Virus Response, Dow Jones Says
- NIH Musters One-Million-Person Study to Track Covid-19’s Spread
- FDA Says No Current Evidence Covid-19 Transmitted Through Food
- Gov. Abbott Reassures Texans as Cases Surge Most Since March
- N.Y. Hospital Visits Hit Low; Calif. Has Fewest New Cases in Weeks
- Behind Florida’s Coronavirus Surge: What the Charts Are Showing
What Else to Know Today
New Risk for 401(k) Retirement Disputes: The coronavirus could spark a new type of claim against 401(k) retirement plans. Companies dealing with Covid-19-related disruptions like remote work and worker furloughs are at risk of getting sued if those disruptions impact investment decisions regarding the company’s retirement plan, and as a result, how well the plan performs, benefits attorneys say. Read more from Lydia Wheeler.
Easy Access to Abortion Pill Called Vital: The FDA should relax restrictions on a drug used for abortion amid the coronavirus outbreak to prevent unnecessary travel, over 100 members of the House said yesterday in a letter to the agency. Under the current rules, a person who wants to use mifepristone to terminate a pregnancy must get it directly from their health-care provider. Mifepristone isn’t available in pharmacies and can’t be mailed directly to patients, Democrats said in a letter to FDA Commissioner Stephen Hahn. Read more from Jacquie Lee.
More Headlines:
- Drugmakers Hold On to Win in Price Advertising Rule Dispute
- New Hampshire Hospitals Lose Request for Patients’ Removal
- Patient Input on New Drugs Given New Weight in FDA Guidance
- Startup Seeks Approval for Inhaler That Would Help Smokers Quit
- Hemp Bombs Gets Pause in CBD False Ad Suit to Await FDA Rule
- Trademarking of Cannabis Additive Under FDA Review Is Rejected
- Valeant’s $1.2 Billion Settlement Recommended for Final Approval
- Medicaid Patients Denied Early Out in Readmissions Case
To contact the reporters on this story: Brandon Lee in Washington at blee@bgov.com; Alex Ruoff in Washington at aruoff@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Zachary Sherwood at zsherwood@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.