HEALTH CARE BRIEFING: Fauci, Health Experts Testify on Reopening
Bloomberg Government subscribers get the stories like this first. Act now and gain unlimited access to everything you need to know. Learn more.
Top U.S. health officials will discuss how to safely restart the economy before a Senate committee Tuesday, with three of the four witnesses — including Anthony Fauci — and the panel’s chairman all appearing remotely because of potential exposure to coronavirus.
The circumstances of the Health Committee hearing, which will include Fauci, the nation’s leading infectious disease expert, and Robert Redfield, director of the Centers for Disease Control and Prevention, accentuates the difficulty the U.S. faces as states move to let businesses reopen and encourage consumers to go out and spend.
Amid the sharpest downturn in U.S. history, President Donald Trump has been pressing to begin relaxing the lockdowns that have shuttered businesses despite warnings from some public health experts that doing so too quickly risks a further spread of the virus.
“I want it to reopen safely, but I want it to reopen,” Trump said at a White House news conference Monday, where he and other administration officials insisted there’s enough testing available to support limited efforts to reopen the country.
But Fauci indicated late Monday that he plans to issue a stern warning against cutting corners on standards set by the administration. These include thresholds such as a “downward trajectory” of documented cases or positive tests “within a 14-day period.” Trump has urged governors to move toward easing their lockdowns even though many states don’t meet those standards.
Citing “the danger of trying to open the country prematurely,” Fauci said in an email to a New York Times reporter, “If we skip over the checkpoints in the guidelines to ‘Open America Again,’ then we risk the danger of multiple outbreaks throughout the country. This will not only result in needless suffering and death, but would actually set us back on our quest to return to normal.”
Plans for the hearing took an abrupt twist on Sunday when Fauci, Redfield and a third witness — Food and Drug Administration Commissioner Stephen Hahn — all said they had come into contact with a member of the White House staff who tested positive for the coronavirus and were in full or partial quarantine. Read more from Laura Litvan.
Stimulus Talks & Economic Pains
Trump Tries to Wait on More Stimulus But Virus Pressure Mounts: Trump and allies are holding off on more coronavirus-related stimulus as his team tracks the impact of some $5 trillion already poured into the economy — and banks on an economic rebound as shutdown measures are eased. But they may find themselves under more pressure to act again, sooner than they expected, if efforts to reopen the economy don’t rapidly bear fruit.
Democrats and a handful of Republicans are seeking a fourth round of stimulus, particularly to help states faced with massive budget deficits. Speaker Nancy Pelosi (D-Calif.), working with Senate Democrats, has been advocating a massive aid package to get the economy restarted. The House may vote on that legislation as soon as Friday. Trump has expressed skepticism over helping states with large pension obligations, while pushing for a payroll tax cut that’s opposed by most Democrats and some Republicans. Read more from Josh Wingrove and Saleha Mohsin.
Democrats Unveil Medicare Loans Bill: Sens. Jeanne Shaheen (D-N.H.) and Michael Bennet (D-Colo.) today will push to include in the next coronavirus response legislation a modification of Medicare’s advanced payment program to assist the thousands of doctors and hospitals that have taken out nearly $100 billion in upfront payments since March. Their bill would allow the federal government to forgive some of the upfront payments due to hardship and lower the interest rates on the upfront payments.
Shaheen and Bennet are the latest to push to lower the interest rate on the Medicare Accelerated and Advance Payments Program, to 1% from as high as 10.25%. The bill would also allow doctors to more easily pay back the upfront payments to Medicare by limiting the portion of Medicare reimbursement that may be withheld by the government to pay down the upfront payment to 25% of the otherwise applicable payment for the service, Shaheen’s office said.
Since expanding the advance payment program in late March, the Centers for Medicare and Medicaid Services have approved $59.6 billion in payments to 21,000 hospitals and other Medicare Part A health-care providers, and $40.4 billion in payments to 24,000 doctors and other Part B providers, CMS said.
Modifying the advance payment program has seen bipartisan support: Shaheen and Sen. Bill Cassidy (R-La.) sent a letter last month to federal health officials asking them to lower the interest rate on the upfront payments, Alex Ruoff reports.
Hospitals Losing $60 Billion a Month: U.S. hospitals are losing an estimated $60.1 billion a month and facing an 113% increase in uninsured patients during the outbreak, according to a new study. The heavy losses reflect a 54% drop in patient visits due primarily to the cancellations of normally more lucrative non-emergency and elective procedures, according to data from 2 million patient encounters in 40 states compiled by Strata Decision Technology, which provides financial analytics for the health-care industry, Tony Pugh reports.
- Meanwhile, after an initial spate of dire warnings of massive costs related to the coronavirus, earnings calls for health insurers so far are showing they did well due to those cancellations of procedures and doctor’s office visits. But analysts are struggling to foresee whether insurers will escape higher costs due to deferred care, or whether they will be hit by a double whammy of big costs for Covid-19, such as post-acute care, and expenses to treat those who didn’t get care when needed. Sara Hansard has more.
Other Letters & Legislation:
- Maloney Asks FEMA to Cover Costs of N.Y. Emergency Response
- Eshoo Urged to Hold Pandemic-Related Mental Health Hearing
- Republican AGs Ask Congress to Probe China Over Coronavirus
- Local Health Agencies Say $7.6 Billion Needed for Contact Tracing
Testing & the Path to Reopening
House Coronavirus Oversight Panel to Focus on U.S. Reopening: A new House panel created to oversee coronavirus relief spending will focus its first briefing on requirements to safely reopen the American economy during the coronavirus pandemic.
Among participants at the hearing on Wednesday will be former Food and Drug Administration Commissioner Scott Gottlieb, one of the authors of a report released in late March by the conservative American Enterprise Institute, “National Coronavirus Response: A Road Map to Reopening.” Gottlieb served as head of the FDA under President Donald Trump until April of last year. A co-author of that report, Mark McClellan, a former commissioner of the FDA and former administrator for the Centers for Medicare and Medicaid Services, also plans to take part in the hearing. Read more from Billy House.
White House to Distribute $11 Billion for Tests: The Trump administration is planning to distribute $11 billion to states for coronavirus testing, according to senior administration officials. The $11 billion is part of the CARES Act stimulus package (Public Law 116-136). It will be distributed under by a formula that reflects states’ burden of Covid-19 as well as population-based estimates, the officials said yesterday. The administration plans to release details about the distribution in the next day or two, the officials said, Emma Court reports.
Trump Declares ‘We Have Prevailed’ on Testing: Trump declared yesterday at a White House news conference on the coronavirus that “we have prevailed,” as U.S. deaths from the virus exceeded 80,000, a remark he later said pertained only to testing for the infection. “Thanks to the courage of our citizens and our aggressive strategy, hundreds of thousands of lives have been saved,” he said.
There have been over 1.3 million cases of Covid-19 in the U.S., according to data compiled by Bloomberg. The U.S. didn’t exceed 100,000 tests performed until March 19, according to data compiled by the Covid Tracking Project, but more than 300,000 tests were conducted daily on Thursday, Friday and Saturday. The Health and Human Services Department’s No. 2 official, Brett Giroir, added that the U.S. should shortly be on pace to conduct 9 million tests per month. Justin Sink and Mario Parker have more.
Trump to Visit Pennsylvania Medical Equipment Supplier Thursday: Trump will visit the factory of Owens and Minor, a medical equipment distributor, in Allentown, Pa., on Thursday, according to a White House official, Ben Livesey and Jordan Fabian report. Trump will tour a distribution center and is expected to discuss the administration’s efforts to utilize the national strategic stockpile to support Covid 19 testing.
Trump Says He Ordered Staff to Wear Masks: Trump said yesterday that he demanded that everyone entering the West Wing wear a face mask, after Vice President Mike Pence’s press secretary tested positive for coronavirus infection last week. The White House made the announcement in a memo addressed to staff yesterday, saying employees didn’t need face coverings while working at their desks. Trump told reporters that he “required” the memo.
“We’ve had just about everybody I’ve seen today has worn a mask,” Trump said. The memo from the White House’s Management Office says: “Staff who sit in the West Wing are not required to wear a facial covering while at their desk if they are appropriately socially distanced from their colleagues.” Justin Sink and Jennifer Jacobs have more.
More Headlines:
- U.S. Virus Cases Rise 1.3%, Lowest Daily Increase Since April 30
- Hopkins Helps Build Army of Virus Tracers With Online Course
- WHO Says Risk of Transmission Will Rise Again With Reopening
- Some New York Regions Ready to Reopen This Week, Cuomo Says
- NYC Virus Lockdown Likely to Continue Into June, de Blasio Says
- Wuhan Sees First New Coronavirus Cases Since Ending Lockdown
- The Software That’s Powering All the Coronavirus Dashboards
- Wuhan to Test 11 Million; Russian Cases Top Spain: Virus Update
- From Mountain Peaks to Arid Deserts, Park Reopenings Spur Debate
Research, Treatment & Coordination
NIH Director Talks Vaccines, Virus Mutation and Clinical Trials: Several vaccines will likely be needed to combat the coronavirus and immunize groups of people in America and abroad, National Institutes of Health Director Francis Collins said in an interview. Collins discussed efforts to develop and manufacture a vaccine, potential mutations and what they mean for immunity, and how inoculations would be tested.
“My expectation is, and I am a bit of an optimist, that we don’t find out that there’s only one of these vaccines that works, but rather two or three of them come through the trials looking as though they’re safe and effective,” Collins said. “They’ll have somewhat different characteristics of where they work best, so we might need to do some matching then of which vaccine goes to which particular population.”
Collins said there’s enough money to rapidly manufacture 100 million vaccine doses by late fall and 300 million before January. The first people to get a vaccine will likely be frontline health workers and those with chronic conditions that put them at greater risk from the illness. Later, Collins said, the U.S. government will have to increase manufacturing to meet global demand, and distribute vaccines to countries particularly hard-hit by the virus. Read more from Riley Griffin.
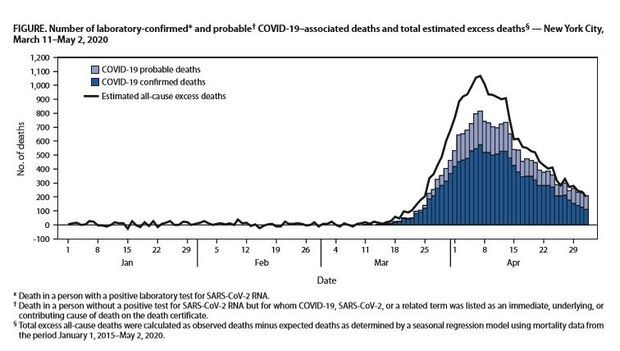
N.Y.C. Saw 24,172 More Deaths Than Normal: New York City had four times the number of deaths as expected during its Covid-19 outbreak, according to a new study, including thousands of excess deaths that might not be attributed directly to the virus but to its effect on the health-care system, city services and other factors. From March 11 through May 2, there was a total of 32,107 deaths, 24,172 more that the city would have expected in that time based on previous trends, according to a report from New York’s Department of Health and Mental Hygiene that was published by the Centers for Disease Control and Prevention. Read more from Michelle Fay Cortez.
Gilead’s Drug Seen in Short Supply for Americans: The U.S. will get less than half of Gilead Sciences’s worldwide donation of 1.5 million vials of its Covid-19 medicine over the next six weeks, which isn’t expected to be enough to treat all the patients who would qualify for it. Gilead is donating about 607,000 vials of its remdesivir in the U.S. during that time frame. That’s enough to treat 78,000 hospitalized patients, according to the Health and Human Services Department.
“Initial supply of remdesivir is likely to be constrained to an even greater degree than we had previously estimated,” RBC analyst Brian Abrahams wrote in a note to clients. He said that he expected 80% of Gilead’s donation to be distributed in the U.S. With less than 50,000 going out in the first two shipments, the rollout was also slower than what he was expecting. Read more from Cristin Flanagan.
FEMA Shifts PPE Buying Power: The Federal Emergency Management Agency is handing over some of its responsibilities for acquiring pandemic supplies to the Defense Logistics Agency, a subset of the Defense Department, according to a FEMA spokesperson. The Defense Logistics Agency will coordinate longer-term purchasing of supplies, like personal protective equipment, the spokesperson said. That agency manages the supply chain for the entire U.S. military and has extensive experience in the area. Read more from Shira Stein.
- Trump will visit the factory of Owens and Minor, a medical equipment distributor, in Allentown, Pa., on Thursday, according to a White House official. Trump will tour a distribution center and is expected to discuss the administration’s efforts to utilize the national strategic stockpile to support Covid 19 testing, Jordan Fabian reports.
States Test Corpses at Nursing Homes: Coast to coast, state governors have intensified efforts to get accurate coronavirus death counts at nursing homes, as investigations suggest far more devastation than initially recorded. Nursing homes account for at least a third of the nation’s 76,000 Covid-19 fatalities, and in 14 states, they are over half the total, according to Kaiser Family Foundation data. Those numbers though are woefully incomplete because 18 states aren’t disclosing such data—and those that are provide varying levels of information. Read more from Elise Young and Keshia Clukey.
More Headlines:
- Infections Near U.S. Meat Plants Rise at Twice the National Rate
- Japan’s Abe Follows Trump in Fast Drug Approvals After Criticism
What Else to Know
Hospitals Offered $1.9 Billion in 2021 Incentives: Medicare would make $1.9 billion available for value-based incentive payments to hospitals in fiscal 2021 under a payment rule proposal released yesterday. Further, Medicare payments to hospitals for uncompensated care would fall by $534 million in fiscal 2021, according to the plan by the Centers for Medicare & Medicaid Services. The proposed changes would update three factors that determine the payments.
The 1,600-page rule proposal also calls for a new Medicare hospital payment category for CAR-T cell therapy treatments, which use a patient’s genetically modified cells to treat certain cancers. The new inpatient payment category would help standardize payment rates for hospitals that offer CAR-T therapy, the CMS said. Read more from Tony Pugh.
More HIV Deaths Foreseen Amid Shortages: The World Health Organization and UNAIDS estimated that a six-month disruption of supplies of antiretroviral therapies could lead to 500,000 extra deaths from HIV in sub-Saharan Africa if countries don’t take action. That’s more than the total number of deaths from HIV in the region in 2018. About 25.7 million people live with HIV in that area, where supplies may be disrupted because HIV services are closed or because of shortages. Some antiretrovirals are being used to treat Covid-19. Read more.
More Headlines:
- Medtech Legal Chief Leaves Amid Company’s Covid-19 Woes
- Doctor’s Defamation Claims Dismissed Under California Law
- J&J, Janssen Face Revived Risperdal Male Breast Growth Suits
To contact the reporters on this story: Brandon Lee in Washington at blee@bgov.com; Alex Ruoff in Washington at aruoff@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Zachary Sherwood at zsherwood@bgov.com; Michaela Ross at mross@bgov.com
Stay informed with more news like this – from the largest team of reporters on Capitol Hill – subscribe to Bloomberg Government today. Learn more.